Aluminum anodizing, an important industrial technique, improves the oxide layer on aluminum surfaces, increasing corrosion resistance and aesthetic appeal. It is mostly utilized in the aerospace, automotive, and electronics industries and entails immersing the material in an electrolytic solution while being charged with electricity.

Aluminum is the second most often used metal in the world. Engineers use aluminums because it is lightweight, corrosion resistant, and inexpensive. However, untreated aluminum has a low wear resistance. When exposed to the environment, it naturally creates a thin layer of aluminum oxide, which gives aluminum its typical corrosion resistance. However, when exposed to other elements in the environment, this naturally occurring oxide film can dissolve
Anodizing aluminum is the solution for offering superior protection. As we will see later in the article, this approach offers other advantages.
Interesting Science Videos
What is Anodizing?
Anodizing is the electrochemical process that turns a metallic surface into a long-lasting, aesthetically appealing finish that resists corrosion. In addition to enhancing metal’s look, anodizing helps shield it from corrosion and wear.
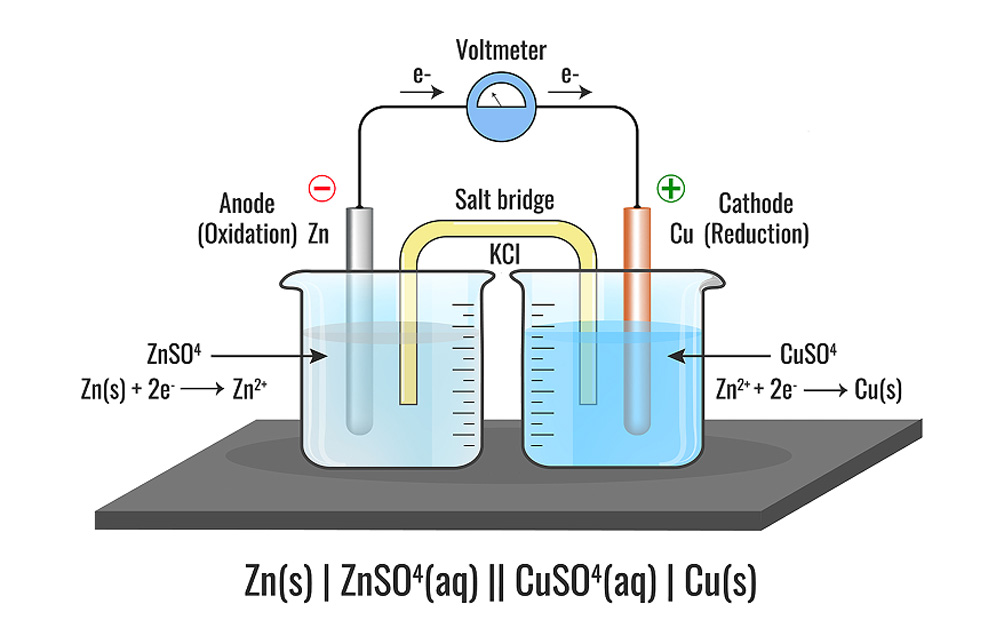
The process of anodizing involves covering an aluminum or other nonferrous metal surface with a precisely regulated, highly adherent coating of oxide. Utilizing an electric current to drive the formation of an oxide layer on the workpiece is how the process operates. By acting as an anode, the workpiece draws in negatively charged oxygen ions. The acidic electrolyte is the source of these oxygen anions.
Electrons released at the cathode, which is typically composed of the aluminum alloy T-6063, though it can also be constructed of other inert, conductive materials, are what drive the creation of the negatively charged ions. At the same time that the negatively charged oxygen ions at the cathode are reduced (take up electrons), positively charged hydrogen ions are produced from the electrolyte. A power supply and wiring to the anode and cathode outside the anodizing tank complete the circuit.
Importance of Anodizing
Anodizing offers metals numerous important advantages. The three main advantages are improved aesthetics, enhanced corrosion protection, and higher wear resistance.
- Anodizing produces a thin oxide coating that coats a metal’s surface, making it far more resilient to abrasion and corrosion.
- Metals are also better suited for painting and dyeing due to the surface that the anodizing process creates, which enables metal surfaces to take on a range of hues.
- Anodizing preserves the metal’s metallic look, in contrast to other metallic finishes.
What is Aluminum Anodizing?
Aluminum anodizing is an electrolytic procedure that thickens the naturally occurring oxide coating on any aluminum surface exposed to air. Aluminum anodizing is a finishing procedure that applies a wear and corrosion-resistant aluminum oxide coating to the surface of aluminum parts. This coating can be tinted after anodizing to provide a decorative effect.
The anodized layer has a porous and organized structure. This porosity allows for easy secondary processing on anodized items, such as dye coloring or surface sealing. The anodized oxide layer works as a barrier, protecting the aluminum from corrosion and wear more effectively than the natural oxide. Aluminum anodizing is a popular finishing procedure since it is affordable, long-lasting, and requires no particular expertise or equipment.
What is Anodized Aluminum?
Anodized aluminum is aluminum that has been anodized to produce a very robust, corrosion-resistant, and visually pleasing surface. An electrochemical technique comprising a succession of tanks is utilized to form an anodic layer on the surface of the aluminum. The layer does not chip, peel, or flake because it is made of metal. Aluminum anodizing makes it three times stronger than regular aluminum and 60% lighter than stainless steel and copper.
- The anodizing process forms a durable aluminum oxide coating that integrates with the underlying aluminum substrate, making raw aluminum tougher and stronger.
- The additional anodic layer is porous, making it ideal for applying dyes, paints, lubricants, and adhesives.
- Aluminum anodizing improves corrosion and wear resistance, allowing it to tolerate extreme weather conditions.
- Anodizing produces a stable aluminum oxide coating that is completely integrated with the underlying aluminum substrate. This layer is tougher and more durable than raw metal. This layer is resistant to chipping, peeling, scratching, and flaking because it is integrated with the metal substrate rather than applied on the surface. This layer’s structure may be very porous, making the anodized item excellent for applying dyes, paints, lubricants, and adhesives. Finally, this layer considerably increases corrosion and weathering resistance in the raw aluminum, allowing it to tolerate hard environments.
- Anodizing does not affect aluminum’s recyclability. Furthermore, unlike electroplating and spraying, this procedure is less harmful to the environment.
Anodized aluminum pieces have a smooth, shiny finish that makes them appealing. Anodizing enables these parts to be stained or colored brilliantly. The tinted film is extremely fade resistant. Consequently, anodized aluminum is frequently employed in decorative and architectural applications.
Aluminum Anodizing Principle
Aluminum anodizing capitalizes on the inherent tendency of aluminum to create a thin oxide coating on its surface. The anodizing process thickens and uniformizes the coating, boosting its protective characteristics in applications that require higher corrosion and wear resistance. Before anodizing, aluminum must be cleaned and the naturally occurring oxide coating removed.
To anodize the clean metal surface, immerse it in an electrically conducting solution. The electrolyte completes the circuit between the aluminum anode and an inert cathode (such as carbon), which can carry electricity but does not react with the electrolyte. The electrolyte is commonly sulfuric or chromic acid, depending on the type of aluminum anodizing, and it aids in the anodic layer’s rate of addition.
An anodizing tank, an anode (positive electrode), and a cathode are used in the process. The anodizing tank receives direct current. Aluminum emits electrons from its surface, resulting in positively charged aluminum ions. Electrons exiting the cathode help to produce negatively charged oxygen ions, which move to the aluminum surface and react with aluminum ions to form a thin coating of aluminum oxide. The thickness of this layer can be adjusted by varying the current density, time, temperature, and electrolyte solution concentration.
The initial layer of the produced oxide, known as the barrier layer, will be continuous and without holes. However, as the oxide layer accumulates, it will obstruct the flow of current. A sequence of attachment sites will form on the barrier layer, resulting in a series of cylindrical pores that are perpendicular to the barrier layer. The current will be dispersed radially outwards from the center of the pore, implying that the subsequent oxide layer will radiate outward until it reaches the oxide layers of the surrounding pores
Materials Required to Anodize Aluminum
- An acid-resistant tank to hold the electrolyte,
- a DC power source to provide current,
- conductive wire to complete the circuit from the power source to the cathode and anode,
- a cathode (typically in the form of a lead sheet),
- cleaned and etched aluminum parts to serve as the anode,
- degreaser,
- etchant, and
- dye for coloring the part after anodizing.
Aluminum Anodizing Process
Anodizing is an electrolytic passivation procedure that thickens the natural oxide deposit on the surface of a component. The resulting coating or layer is strong, stable, and improves aluminum’s natural corrosion resistance. Aluminum anodizing is a controlled oxidation technique that uses an acidic electrolyte bath in tanks with direct electrical current (DC) applied to anodes and cathodes.
The anode is made up of anodized aluminum pieces. The electrical circuit’s negative component is supplied by a cathode plate or rod made of platinum, stainless steel, lead, or carbon. As voltage is given to the circuit, an aluminum component loses positive ions and attracts negative ones, causing a coating of aluminum oxide to develop on the aluminum parts.
An anodizing operation might be batch or continuous. Batch anodizing involves placing the pieces in a rack and immersing them in a number of baths. The components are collected at the end of the process. Batch anodizing is used in cookware, castings, and bent or machined pieces. Continuous or coil anodizing involves unwinding the pre-rolled material and continually running it through the manufacturing phases. Once the stages are done, the material is coiled and delivered to the customer. Continuous anodizing is used for less deformable objects such as wires, plates, sheets, and foils.
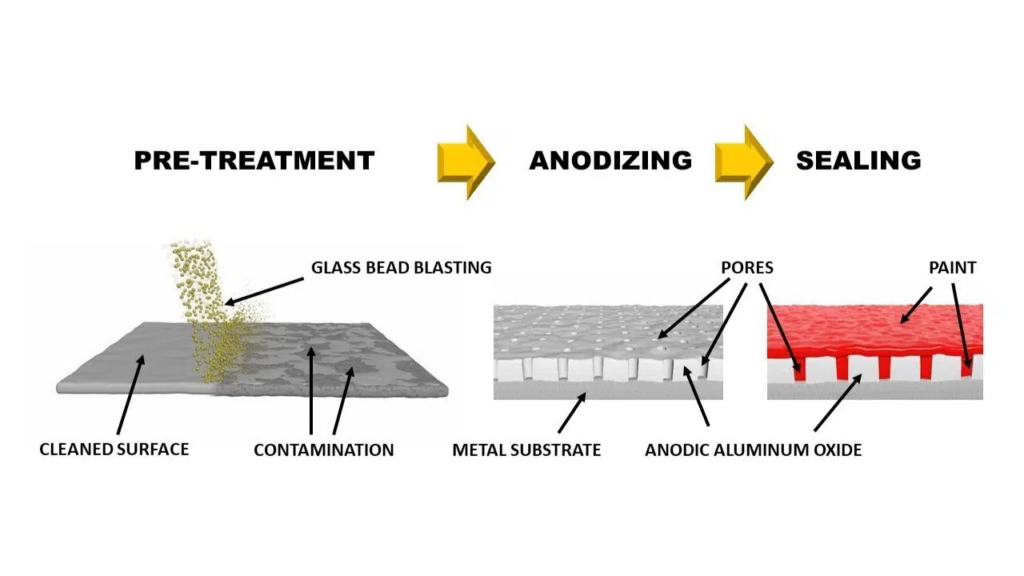
An aluminum anodizing operation has four stages:
Pre-treatment
The pre-treatment stage is critical since it influences the anodized part’s finishing quality and final look. It entails eliminating pollutants such as dirt and grease from the raw aluminum part surface that may impede the process, as well as removing minor surface flaws. In addition, machining techniques such as drilling, cutting, and welding must be completed prior to this stage.
Chemical or mechanical procedures can be used as a pre-anodizing treatment:
- Chemical Pretreatment
- Chemical pre-treatment entails employing a variety of chemical solutions to remove dirt and grease (by an acid or alkali cleanser), surface oxides, and heat-treated scales (via a deoxidizing agent).
- After that, an etching or brightening procedure is conducted to vary the texture of the surface, resulting in a distinct appearance.
- An etching procedure results in a dull or matte surface. This procedure removes a uniform layer from the aluminum part’s surface, thereby minimizing small surface imperfections. Etching is done by immersing the pieces in either hot sodium hydroxide or trisodium phosphate (alkali etching) or aqueous ammonium bifluoride (acidic etching).
- A brightening step creates a shiny or mirror-like finish. This procedure flattens and smoothes down the small peaks and flaws on the metal part’s surface. Thus, roughness is decreased, resulting in a highly reflecting surface. To brighten the pieces, immerse them in a phosphoric or nitric acid bath. Additives are added to the acid bath to improve its brightening capabilities and prevent hazardous emissions.
- Mechanical Pretreatment
- Mechanical pre-treatment techniques include abrasive polishing, sandblasting, and shot peening to expose. Sandblasting and shot peening can help increase the fatigue resistance, hardness, and coating adherence of aluminum parts. For the coating to be stable, it must have good adhesive properties.
Electrolysis
Anodizing operations revolve around electrolysis. During this stage, the aluminum component is immersed in an electrolytic solution bath containing many positively and negatively charged ions. The aluminum portion and the cathode are linked to the DC power supply’s positive and negative terminals, respectively. When DC is applied to close the circuit, the part becomes positively charged as electrons are pulled away from its surface. These electrons go through the electrolyte solution to the cathode contact where they react with hydrogen ions to produce hydrogen gas. Aluminum oxide (Al₂O₃) layer forms when aluminum cations on the surface react with water.
The chemical reactions are summarized below:
Reactions at the Anode
Al → Al3+ + 3e–
2Al3+ + 3O2- → Al2O3
2Al3+ + 3OH → Al2O3 + 3H+
Reactions at the Cathode
2H+ + 2e– → H2(g)
Overall Reaction
2Al + 3H2O → Al2O3 + 6H+ + 6e–
Aluminum Anodizing Process MIL-A-8625 specification
According to the MIL-A-8625 specification, there are four distinct types of aluminum anodizing procedures. These classes produce distinct features.
Chromic acid anodizing
Chromic acid anodizing, also known as type I anodizing, produces the thinnest anodic coat of the three main varieties, often measuring.00002″-.0001″ (20 to 100 microinches) per surface. While thin, when properly sealed, chromic anodize provides the same corrosion protection to aluminum as thicker sulfuric and hardcoat anodize.
Chromic anodize is substantially grayer in hue and absorbs less color when dyed. This limits the decorative applications of chromic acid anodize; however, it can be colored black and used as a non-reflective, protective coating on optical component housings. Even black dyed chromic anodize has a softer (grayer) look than typical sulfuric black anodize.
To induce chromic anodize to accept black dye, the temperature of the chromic acid must be raised; thus, it cannot be done every day and must be timed correctly.
Chromic Acid Anodize Features:
- Good for tight tolerance parts: does not change dimensions.
- Can be black colored; other colors are not practicable.
- Suitable for bonding Non-conducting
- Ideal for welded parts and assemblies.
Chromic Acid Anodizing Applications:
- Precision Machined components.
- Aerospace Components
- Welded parts and assemblies
Boric-sulfuric acid anodizing (BSAA)
Boric-Sulfuric Acid Anodize (BSAA) is an alternative to chromic acid anodize (CAA) due to environmental, worker safety, and health issues, as well as the associated expenses of continuing to employ hexavalent chromium-bearing procedures like CAA.
The primary uses are aircraft and aerospace components. It is covered under MIL-A-8625, Type Ic, and aerospace requirements such as Boeing’s BAC 5632. It is used to prevent corrosion and promote paint adherence. Paint adhesion is equal to or superior to chromic acid, and the method is less energy-intensive than chrome-based techniques.
Boric-Sulfuric Acid Anodization Features:
- Suitable for parts with tight tolerances: will not modify the measurements.
- corrosion protection
- Suitable for bonding Non-conducting
Boric-Sulfuric Acid Anodize Applications:
- Precision machined components
- Aerospace/Aircraft Components
- Sulfuric acid anodize is used as a paint or primer basis.
Sulfuric acid anodize
The sulfuric acid technique is the most used way of anodizing. The sulfuric acid anodize technique produces films that are.0001″-.001″ thick. The coating’s overall thickness is 67% penetration in the substrate and 33% growth over the part’s initial dimensions. It is particularly well suited to applications that demand hardness and abrasion resistance.
However, where parts are subjected to high stress (such as airplane parts), the presence of corrosive acid residue is undesirable. The porous character of sulfuric acid films prior to sealing is particularly useful in the manufacture of colorful surface finishes on aluminum and related alloys.
The porous aluminum oxide readily absorbs dyes, and subsequent sealing helps to avoid color loss in service. Although dyed anodised films are reasonably colorfast, prolonged exposure to direct sunshine causes them to fade.
Some of the colors include black, red, blue, green, urban grey, coyote brown, and gold. To create a matte (non-reflective) appearance, parts might be chemically or mechanically treated before being anodized.
Sulfuric Acid Anodizing Benefits:
- Less expensive than other methods of anodize in terms of chemicals, heating, power usage, and time required to achieve the desired thickness.
- Additional alloys can be finished.
- Harder than chromic anodize.
- A clearer finish allows for more color options when dying.
- Waste treatment is simpler than chromic anodization, which helps to minimize costs.
Sulfuric Acid Anodizing Applications:
- Optical components
- Hydraulic valve bodies
- Military weapons
- Computer and electronic enclosures.
- Mechanical hardware
Hard anodize (hardcoat)
While hardcoat anodizing is typically done with a sulfuric acid-based electrolyte, it is substantially thicker and denser than traditional sulfuric anodizing. Hardcoat is intended for aluminum components that will be subjected to heavy wear applications requiring superior abrasion resistance, as well as corrosive environments requiring a thicker, harder, more lasting coating.
It can also be useful in situations when increased electrical insulation is required. Because hardcoat anodize can be produced up to several thousandths in some circumstances, it is a viable option for salvaging old or mis-machined components.
Hard Anodize Features:
- Improved wear resistance.
- Non-conductive
- Can you fix damaged surfaces on aluminum?
- Improve the part surface for slide applications.
- Can be black colored; other hues are less ornamental.
- Finishes are harder than tool steel.
- Can be ground or lapped.
Hard anodize applications
- Valves and Pistons
- Sliding Parts
- Hinge Mechanisms
- Cams
- Gears
- Swivel joints
- Insulation Plates
- Blast Shields
Titanium anodizing
Titanium Anodizing is the controlled formation of an oxide coating on the surface of titanium objects. Titanium anodize is utilized for a variety of purposes, including part identification in the medical device industry and corrosion control and bonding in aircraft. Depending on the grade of titanium alloy, it can be anodized in a variety of colors, however Anoplate only offers a blue-gray finish.
Anoplate has been depended on to provide this finish in accordance with AMS 2488 Type II and a range of unique customer demands.
Titanium Anodizing Features:
- Used for coloring titanium.
- Used to look for stress cracks in the base material.
- Improved corrosion resistance.
- Improve the adherence of dry film lubricants or paint applications.
Titanium Anodizing Applications:
- Airplane parts
- Medical devices
- Spacecraft components
Other Aluminum Anodizing Process
- Boric-Sulfuric Acid Anodization: Boric-sulfuric acid anodizing (BSAA) is an alternative to Type I anodizing due to environmental and safety issues. As a Type I anodizing process, BSAA provides exceptional paint, lubricant, and adhesive adherence. It also provides strong corrosion resistance and is appropriate for products with close tolerances. BSAA is also used extensively in the manufacture of aviation and aerospace parts.
- Phosphoric Acid Anodizing: Phosphoric acid anodizing, often known as PAA or the Boeing Process, is an alternative to Type I anodizing that uses phosphoric acid to form oxide films. The rough morphology of PAA-created films contains protrusions and whiskers on the surface, which gives the oxide film good adhesive properties. This film can also tolerate high humidity. As a result, PAA can be utilized to prepare the aluminum surface before applying bonding primer. PAA is widely utilized in structural adhesive bonding.
- Thin-Film Sulfuric Acid Anodization (Type IIb): Thin-film sulfuric acid anodizing (TFSAA) use a sulfuric acid-based electrolyte bath of lower concentration than Type II anodizing. As a result, TFSAA yields a thinner oxide film than Type II and III anodizing. As a result, it can be regarded an alternative to Type I anodization.
- Clear anodizing: Clear anodizing is the process of anodizing an item using sulfuric acid and then sealing it in a hot water bath. The finished product is not transparent; instead, this method is employed to apply a homogeneous, translucent film to the aluminum portion, increasing its aesthetic value. The anodized item is often left undyed, with the color created determined by the thickness of the oxide deposit. Clear anodizing is commonly used to polish vehicle trim, window and door frames, photographic plates, and extrusion profiles.
- Bright Dip Anodizing: Bright dip anodizing is a pre-treatment with a phosphoric and sulfuric acid mixture that produces a glossy, highly reflecting finish. The pretreatment is followed by Type II anodization. The anodized component is then submerged in a coloring pigment before the porous film is sealed off. The look depends on the quality of aluminum alloy. Nonetheless, vivid dip anodizing improves the overall appearance of the item.
- Black anodizing: Black anodizing starts with a regular anodizing stage and then colors the anodised object using an organic or inorganic black dye. Black dyes are one of the dyes designed specifically for coloring aluminum parts. Inorganic dyes, such as ferric ammonium oxalate, provide a more lightfast finish than organic colors. Lightfastness refers to a finish’s ability to resist fading when exposed to light. Electrodeposition of a coloring metal to create a black anodized finish is also possible.
- Color Anodizing: Color anodizing consists of a regular anodizing step followed by dip coloring using organic dyes. Organic dye colors vary more than inorganic dye hues. Color anodizing is mostly employed in aesthetic applications. However, organic dyes result in a less lightfast finish than black anodized parts.
- Sealing: The final stage of this process is to seal the aluminum anodizing operation. It binds the absorbed dye, lubricant, or glue to the porous film. It keeps the porous layer from corroding, staining, and absorbing undesirable compounds. It also prevents the color from fading. It is performed by treating the part with a sealing agent that either shuts or reduces the film’s pore opening sizes. Due to the sensitivity of the oxide film, sealing must be done promptly following the coloring stage.
Benefits of Aluminum Anodizing
Durability: Most anodized items have an unusually extended life cycle and provide significant economic benefits through maintenance and operating savings. Anodizing is a reactive finish that is merged with the underlying aluminum, resulting in total bonding and unparalleled adhesion.
Color Stability: Most anodized items have an unusually extended life cycle and provide significant economic benefits through maintenance and operating savings. Anodizing is a reactive finish that is merged with the underlying aluminum, resulting in total bonding and unparalleled adhesion.
Easy to Maintain: Scars and wear from fabrication, handling, installation, frequent surface dirt cleaning, and use are almost non-existent. An anodized surface can typically be restored to its former appearance by rinsing or scrubbing with mild soap and water. More tough deposits can be removed using mild abrasive cleansers.
No chance of filiform corrosion: Filiform corrosion is an attack on the hidden interlayer between the metal and the surface finish, causing corrosion to spread behind the finish. Anodizing incorporates the oxide (anodic) layer into the aluminum. There is no interlayer between the metal and the protective oxide layer.
As a result, the finish will be free of filiform corrosion. If the surface is perforated or damaged, the aluminum will heal itself naturally through oxidation.
Aesthetics: Anodizing provides a huge range of gloss and color options while minimizing or eliminating color variances. Unlike other coatings, anodizing allows aluminum to retain its metallic appearance.
Cost: Lower original finishing costs combined with lower maintenance expenses lead to higher long-term value.
Optimal coverage: Anodizing, as an immersion technique, provides more consistent surface covering, particularly on extruded portions. Spray paint methods, particularly powder coating, frequently leave the non-visible surface uncoated.
Highly impenetrable anodic layer: A well sealed anodic layer is impenetrable. Furthermore, when extreme external temperature fluctuations occur, the anodic layer is not subjected to detrimental physical changes and subsequent embrittlement. These alterations can occur when an organic covering is exposed to temperature cycles above and below its glass transition temperature.
Health & Safety: Anodizing is a safe process that does not impact human health. An anodised finish is chemically stable, does not disintegrate, is non-toxic, and heat-resistant to the melting point of aluminum (1,221 degrees Fahrenheit). Because the anodizing process reinforces a naturally occurring oxide reaction, it is non-hazardous and emits no harmful or dangerous byproducts.
Limitations of Aluminum Anodizing
Here are some things to remember:
- Only specified grades of aluminum can be used for this technique.
- It cannot be used on stainless steel, which is resistant to mortar, salt, chlorine, and marine environments.
- The most expensive solution for small numbers (needs a higher-grade alloy in addition to the high setup expenses).
- Thick anodic coatings, particularly those incorporating Type III techniques, may diminish component fatigue life.
- Geometric modifications must be addressed while anodizing any parts. This is essential for Type II and III processes, but it may not be necessary for some Type I.
- Color matching can be complicated when dealing with various batches and extremely challenging when working with different vendors.
- This process uses the base metal, thus color differences may occur. In high quality metals, a 95% match is feasible between batches; in low grade metals, no match is possible.
- In comparison to plating, it is more difficult to replicate consistency between batches.
- To ensure total corrosion protection, the anodic layer’s pores may need to be sealed.
- Type III hard coatings’ abrasion resistance decreases as thickness exceeds 0.003″.
- The anodizing process may have varied effects on different alloys. For example, metals with 2% or more copper content are often less wear-resistant than other alloys when tested for MIL Spec Type III coatings. This means that Type III hard coat on 2000 series aluminum and some 7000 series will be less wear-resistant than 6061 with a hard coat.
What Type of Anodizing Is Best?
It is not easy to choose the best sort of anodizing and understand how it works. You should choose the anodizing technique based on the numerous applications for aluminum parts. A highly qualified business may recommend the optimum type of anodizing for your project. Comparing different types of anodizing allows you to select the best anodized aluminum parts.
- Type I has specialized features, such as strong corrosion resistance, and employs chromic acid to form a thin film on the surface of parts. Type I is suitable for manufacturing airplane parts.
- Type II anodizing is most commonly employed in architectural and aeronautical applications. Type II coats items with thick anodized layers using sulfuric acid rather than chromic acid. Type II has modest wear resistance and applies a thick layer of sulfuric acid to the surface of the metal portion.
- Type III is ideal for components that can endure chemical exposure and high temperatures. Type III has the same characteristics as type II, however the results vary. Type III forms a layer of corrosion and is used to produce strong metal products.
Where is Anodized Aluminum Used?
Architecture: Anodized aluminum will not corrode, tarnish, or weather, making it an appealing material for gorgeous, modern structures and construction exteriors or interiors. Because anodized aluminum can be customized in any color, gloss, or texture, it is an appealing, lightweight, and cost-effective solution for interior finishes such as ceiling panels, retail signage, and elevator panels.
Transportation: Anodized aluminum is three times harder than the raw material and can function at any pace. From commercial airplane interiors to rail car panels to the external brilliant trim on sports cars, anodized aluminum provides the durability and weather resistance that transportation vehicles require, as well as the excellent appearance that clients seek.
Consumer goods: Consumers seek visually appealing, long-lasting products that reflect their aesthetics and lifestyle. Anodized metal can provide you with any look, texture, or color you desire. From kitchen backsplash panels to microwaves, coffee machines, and other consumer goods, anodized aluminum provides the versatility and quality that your customers expect.
Anodized aluminum may be a lovely addition to any project or product, whether it’s impressive buildings and structures, artwork, the latest line of luxury vehicles, or high-end appliances. The anodizing process is environmentally sustainable, resulting in a finish of exceptional and dynamic beauty, endurance, and durability.
References
- https://www.iqsdirectory.com/articles/aluminum-anodizing/anodized-aluminum.html
- https://www.xometry.com/resources/machining/aluminum-anodizing/
- https://fractory.com/aluminium-anodising/#Aluminium_Anodising_Process
- https://www.fictiv.com/articles/aluminum-anodizing-all-you-need-to-know
- https://www.anodizing.org/page/anodizing-benefits
- https://www.unitedanodisers.com/about-anodising/key-advantages-of-anodising.html
- https://www.pfonline.com/articles/aluminum-anodizing
- https://aerospacemetalsllc.com/the-complete-guide-to-anodizing-aluminum-parts/
- https://waykenrm.com/blogs/anodizing-aluminum/
- https://www.lorin.com/what-is-anodized-aluminum/
- https://www.anoplate.com/finishes/anodizing/
