Life on Earth depends on liquid water. Most likely, 60% of your body weight is water. The body uses water for a variety of essential processes, including chemical reactions, waste removal, and nutrition transportation. Water serves the same functions in plants, which are mostly made of it. Water is used by all known life to carry out its essential tasks. Although the structure of water molecules is straightforward (H2O), the compound’s physical and chemical properties are incredibly complex and not typical of most substances found on Earth. Undoubtedly, the biological properties of water explain its greater significance but we here will be more focused on the properties of water from a chemical perspective such as structure, physical characteristics, and chemical properties.

Interesting Science Videos
Structure of Water
Two hydrogen atoms that are individually connected to an oxygen atom by a single chemical bond make up the water molecule. Water is a polar molecule that always has an electron-rich oxygen atom and an electron-poor hydrogen atom. This causes the water molecules to interact through hydrogen bonds. The structure of water changes greatly depending on its physical condition.
![Physical State of Water [Image Source: Britannica] Properties of Water](https://scienceinfo.com/wp-content/uploads/2023/02/image-11.png)
Structure of water in the gas phase
Water in the gas phase is made up of solitary H2O molecules. Each molecule is bent at 105 degrees. Around the oxygen atom, there is a strong negative charge. Protons are somewhat positively charged. According to the electron density map, the electron density is around ten times higher around oxygen than it is around hydrogen atoms. Both the IR and microwave spectra of water vapor can be used to identify and measure it.
Structure of water in the solid phase
Each oxygen atom in the solid-state (ice) is surrounded by four hydrogen atoms, two of which are covalently bound to the oxygen atom while the other two (at greater distances) are hydrogen-bonded to the oxygen atom’s unshared electron pairs. This results in a highly ordered yet flexible structure’
Ice’s open structure makes it less dense than liquid water, which has a partially broken-down ordered structure and water molecules that are (on average) closer together. Water can form a variety of structures when it freezes, depending on the circumstances. The position of the oxygen atoms in a cubic, closely packed lattice of water is depicted in the diagram on the right. A hydrogen atom sits in the middle of the lines joining one oxygen atom to the next. In the ice structure, the bonds are rigid.
Structure of water in Liquid State
Two hydrogen atoms are combined into one water molecule via a single chemical interaction with an oxygen atom. Protons make up the complete nucleus of the majority of hydrogen atoms. Deuterium and tritium, two isotopic forms of water, have atomic nuclei that also contain one and two neutrons, respectively. Deuterium oxide (D2O), commonly referred to as heavy water, is utilized in nuclear reactors and chemical research as a neutron moderator.
The tetrahedral and “ring-and-chain” type structures that makeup water’s structure are dynamically mixed together, with a small preference for the former. Each water molecule creates roughly three hydrogen bonds with the water molecules around it on average.
In water molecules, hydrogen atoms are attracted to regions with high electron densities and can establish weak interactions with those regions which are known as hydrogen bonds. This indicates that the oxygen atoms of a nearby water molecule are attracted to the nonbonding electron pairs of the hydrogen atoms of one water molecule. It is believed that the aggregates of water molecules that continuously form and reform make up the structure of liquid water. This short-range order explains other peculiar characteristics of water, such as its high viscosity and surface tension.
Physical Properties of Water
There are numerous significant physical properties of water. Although these properties are well-known due to water’s ubiquitous existence, the majority of water’s physical properties are rather unusual. Water has an exceptionally high viscosity, surface tension, heat of vaporization, and entropy of vaporization values given the low molar mass of its constituent molecules. Solid water is less dense than liquid water, which is relatively rare among common substances and is due to the open structure of ice that promotes maximum hydrogen bonding.
Water is a tasteless and colorless liquid. Due to the many hydrogen connections between water molecules, the condensed form of the substance has peculiar features. High melting and boiling points are also a result of this. Water has greater specific heat, thermal conductivity, surface tension, dipole moment, and other properties than other liquids. Its importance to the biosphere results from these characteristics. Water is a great solvent; thus, it facilitates the movement of the ions and molecules needed for metabolism. Because of its high latent heat of vaporization, it aids in controlling body temperature.
Physical Appearance: The water appears to be odorless and colorless. Since the liquid is a transparent material, anything behind the water may be seen clearly.
Boiling and Melting Point: The boiling point of water is 100°C and the Melting point is zero degrees Celsius. In contrast to the low boiling points of hydrogen telluride and hydrogen sulfide, hydrogen (the next hydride) has a very high boiling point. This is caused by the water molecule’s extraordinarily strong hydrogen bonds. They require a significant amount of energy to break and start boiling. Water takes a very long time to freeze (or even boil), which is essential for the survival of our ecosystem.
Density: One unique property of water is that in its solid state, it is dense. Up to 4°C water’s density does increase on cooling. But after that point water becomes less dense. This is why ice floats in water. Water becomes denser as it cools up to 4°C. After that, though, water becomes less dense.
Viscosity: Fluids with a high barrier to flow are said to have a high viscosity. When compared to other substances with similar structures, water is viscous, contrary to what we typically think of when we think of viscous liquids like honey or motor oil. Generally speaking, liquids with greater intermolecular connections are more viscous than those with weaker contacts.
Compressibility: Both pressure and temperature have an impact on compressibility. Water has such low compressibility that it is frequently thought to be incompressible. Water in deep waters under high pressure can only lose 1.8% of its volume due to limited compressibility.
Adhesion and Cohesion: Water molecules regularly create and break hydrogen bonds with other water molecules. Water molecules’ capacity to adhere to one another is known as cohesion. Water has great adhesion, or the capacity to stick to other surfaces, thanks to its polarity. Stronger than cohesive forces are adhesive forces.
Surface Tension: Additionally, water has a high surface tension of 71.99 mN/m at 25 degrees due to hydrogen bonding. Since the surface tension is so strong, insects may traverse the water. Water’s cohesive qualities cause surface tension. The strong surface tension of water is demonstrated by water droplets and water rising past a glass’s rim.
Specific Heat Capacity: The amount of heat needed to increase a substance’s temperature by one degree Celsius per gram is known as its specific heat capacity. Water has a specific heat capacity of 1 cal/(g°C). Compared to other common materials, water has a substantially larger specific heat capacity. For comparison, metal has a specific heat capacity of about 0.2 cal/(g°C) while oil has a specific heat capacity of about 0.5 cal/(g°C). This indicates that raising the temperature of water requires significantly more heat than raising the temperature of oil or aluminum. The earth’s temperature is kept temperate by the high specific heat of water because it absorbs heat during the day and releases it gradually at night.
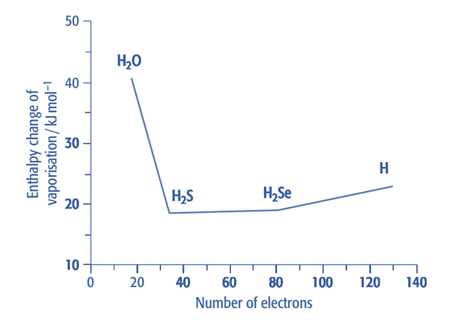
Enthalpy change of vaporization: Unexpectedly, the enthalpy change of vaporization for water is substantially higher. This is a result of the substantial hydrogen bonding. The increase in enthalpy change of vaporization from H2S to H2Te is caused by the Group 16 atoms having more electrons as we move down the group. As molecules become larger, as a result, van der Waals forces rise. We would anticipate that water’s enthalpy change would be around 17 kJmol-1 if van der Waals forces were the only interactions between its molecules. However, the enthalpy change of water vaporization is substantially larger. This is a result of the substantial hydrogen bonding in water.
Chemical Properties of Water
Amphoteric property: Water’s amphoteric property is one of its distinctive characteristics. Water is neither acidic nor basic, but it functions as both. This is because of its ability to both donate and accept protons. It serves as a base for acids that are stronger than water. And it behaves like an acid toward bases that are stronger than it. These two subsequent reactions demonstrate this amphoteric character.
H2O (l) + HCl (aq) ⇌ H3O+ + Cl–
H2O (l) + NH3 (aq) ⇌ NH4+ + OH–
Redox Reactions: Electropositive elements reduce water to a hydrogen molecule in redox reactions. Water is therefore a plentiful source of hydrogen. During the photosynthesis process, water is oxidized to O2. Due to its ability to be both reduced and oxidized, water is particularly helpful in redox processes.
Liquid water and active metals like sodium react violently in an exothermic (heat-producing) process that emits burning hydrogen gas.
2Na(s) + 2H2O(l) → 2Na+(aq) + 2OH−(aq) + H2(g)
Hydrolysis Reaction: Water has a very strong hydrating propensity because of its high dielectric constant (hydrolysis process). A wide variety of ionic compounds are dissolved by it. Certain covalent and ionic molecules are hydrolyzed by water.
SiCl4 + 2H2O → SiO2 + 4HCl
Water: “Universal Solvent” – Most Important Properties of Water
Water interacts best with other polar molecules, including itself, because it is a polar molecule. This is due to the phenomena of attraction between opposite charges; each water molecule contains both a positive and a negative charge, and as a result, each side is drawn to molecules with the opposite charge. Water may link to other polar molecules in its environment, such as other water molecules, by forming relatively strong interactions, or bonds. In this scenario, the positive hydrogen of one water molecule will combine with the adjacent molecule’s negative oxygen, attracting its own hydrogens to the subsequent oxygen, and so on. Water molecules can establish bonds with and surround both the positive and negative areas of biological molecules because most of them are polar and have some electrical asymmetry.
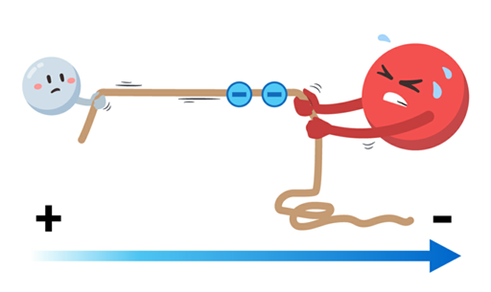
The term “universal solvent” refers to water’s enormous capacity to dissolve a wide range of molecules, and it is this capacity that makes water such a priceless life-sustaining agent. Water’s function as a solvent aids cells in the transfer and utilization of chemicals like oxygen and nutrients on a biological level. Blood and other water-based fluids assist in transporting molecules to the required regions. As a result, water’s function as a solvent makes it easier for molecules like oxygen to go through the body during respiration and has a significant impact on how quickly medications can reach their intended targets in the body.
Information Related to Water: Properties of Water
| PubChem CID | 962 |
| Molecular Formula | H2O |
| Molecular Weight | 18.015 |
| Boiling Point | 212 °F at 760 mmHg 99.974 °C |
| Melting Point | 32 °F / 0 °C |
| Solubility | Completely miscible, Water dissolves some amount of virtually every solid or gas with which it comes in contact, very soluble in ethanol, methanol, acetone |
| Density | 0.9950 g/cu cm at 25 °C Solid: 0.9167 g/ml at 0 °C Liquid: 0.961893 g/mL at 95 °C 0.9970474 g/mL at 25 °C 0.9998396 g/mL at 0 °C |
| Viscosity | Dynamic viscosity: 0.8949 cP at 25 °C Kinematic viscosity: 0.8976 cP at 25 °C |
| Heat of Vaporization | 9.717 kcal/mole |
| Surface Tension | 71.97 dyne/cm at 25 °C |
| Refractive Index | 1.33 |
| Allotropic forms | ice (solid) and steam (vapor) |
| Crystal structure | Hexagonal |
| Molecular shape | Bent |
| Point group | C2v |
| Specific heat capacity (C) | 75.375 ± 0.05 J/mol·K |
| Std enthalpy of formation (ΔfHo298) | -285.83 ± 0.040 kJ/mol |
| Std molar entropy (So298) | 69.95 ± 0.03 J/mol·K |
References
- Chaplin, Martin (2019). “Structure and Properties of Water in its Various States”. Encyclopedia of Water. Wiley Online Library 2019. pp. 1–19. doi:10.1002/9781119300762.wsts0002. ISBN 9781119300755. S2CID 213738895
- Lewis, William C.M.; Rice, James (1922). A System of Physical Chemistry. Longmans, Green and Co.
- Lide, David R. (2003-06-19). CRC Handbook of Chemistry and Physics, 84th Edition. CRC Handbook. CRC Press. ISBN 9780849304842.
- Reece, Jane B.; Urry, Lisa A.; Cain, Michael L.; Wasserman, Steven A.; Minorsky, Peter V.; Jackson, Robert B. (2013-11-10). Campbell Biology (10th ed.). Boston, Mass.: Pearson. ISBN 9780321775658.
- Riddick, John (1970). Organic Solvents Physical Properties and Methods of Purification. Techniques of Chemistry. Wiley-Interscience. ISBN 978-0471927266.
- science/water/Structures-of-ice
- https://byjus.com/chemistry/physical-and-chemical-properties-of-water/
- https://www.geeksforgeeks.org/structure-and-properties-of-water/
- https://sitn.hms.harvard.edu/uncategorized/2019/biological-roles-of-water-why-is-water-necessary-for-life/
- Weingärtner, Hermann; Teermann, Ilka; Borchers, Ulrich; Balsaa, Peter; Lutze, Holger V.; Schmidt, Torsten C.; Franck, Ernst Ulrich; Wiegand, Gabriele; Dahmen, Nicolaus; Schwedt, Georg; Frimmel, Fritz H.; Gordalla, Birgit C. (2016). “Water, 1. Properties, Analysis, and Hydrological Cycle”. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.
- https://www.chemistryworld.com/features/the-weirdness-of-water/4011260.article
