
Aromaticity is the property of an organic compound. Aromatic compounds have high stability. In 1931, German chemist and physicist Erich Hückel developed a theory to help determine if a planar ring molecule would have aromatic characteristics. According to his rule, aromatic molecules have 4n+2 π electrons and are cyclic and planar in shape. This rule became known as Hückel’s Rule.
“Aromaticity” refers to the property of a cyclic, planar molecule with a ring of resonance bonds more stable than other geometric or connective configurations of the same atoms. To be an Aromatic Compound or possess Aromaticity, compounds must satisfy the following four conditions:
- The molecule’s structure must be cyclic.
- All atoms in the cyclic ring must be conjugated. It will provide a cyclic ring-delocalized pi-electron system. As a result, we can conclude that each atom in the cyclic ring must have an empty p orbital and be capable of resonance.
- Huckel’s Rule must be followed by all compounds.
The molecule should be plane or flat. Compounds that follow the above four Rules of - Aromaticity is often flat as they have high potential energy.
Thus, Huckel’s Rule is one of the requirements that Aromatic Compounds must meet in order to be considered Aromatic.
Interesting Science Videos
Huckel Rule: Aromaticity
In 1931, Eric Huckel, a German physicist and chemist, proposed a theory that identified Aromatic characteristics in Planar Ring Molecules. According to Huckel’s Rule, a cyclic, planar molecule with 4n + 2 π electrons is Aromatic. Huckel’s Rule states that all planar aromatic compounds must have 4n+2 π electrons, where n is an integer (e.g., 0, 1, 2, 3, 4). This Rule predicts whether a planar ring compound will have aromatic qualities or not.
Huckels Rule is a collection of algorithms that determines whether the molecule is aromatic, anti-aromatic, or non-aromatic by combining the number of π electrons and the physical structure of the ring system.
Planar monocyclic molecules with 4n π-electrons are known as antiaromatic and are generally unstable. A compound’s stability is proportional to its delocalization energy (resonance energy). The resonance energy can be determined using Huckel Molecular Orbital Theory (HMOT). Antiaromatic substances are unstable because they have zero resonance energy.
| Aromatic compounds | Antiaromatic compounds | Non Aromatic Compounds |
| They are cyclic. | They are cyclic. | They are cyclic. |
| All atoms are SP2 hybridized. | All atoms are SP2 hybridized. | They have SP3 hybridized atoms. |
| They are planar. | They are planar. | They are non-planar. |
| They contain 4n+2 π electrons. | They contain 4n π electrons. | |
| E.g. Benzene | E.g. Cyclobutadiene | E.g. Cyclopropene |
Aromatic compounds
Aromatic compounds are often more chemically stable than comparable non-aromatic ones. A molecule that has the potential to be aromatic will tend to become aromatic, and the extra stability modifies the molecule’s chemistry. Unlike carbon–carbon double bonds, which experience electrophilic addition reactions, aromatic compounds undergo nucleophilic and electrophilic aromatic substitution reactions.
When a magnetic field is present, the circulating π-electrons in an aromatic molecule generate an aromatic ring current, resulting in an extra magnetic field. This process is crucial for nuclear magnetic resonance.
Aromatic ring protons have a significantly lower NMR signal than non-aromatic sp 2 carbons. This is an effective technique to determine aromaticity.
Aromatic molecules can interact in a process known as “π-π stacking.” The π systems form two parallel rings that overlap in a “face-to-face” arrangement. Aromatic molecules can interact in an “edge-to-face” orientation. The positive charge of substituents on one molecule’s ring atoms attracts the negative charge of another’s aromatic system.
Aromatic compound with a single-ring
Benzene is the most common aromatic compound with one ring. Planar, completely conjugated rings larger than benzene with 4n + 2 π electrons are aromatic.
Annulenes are hydrocarbons composed of a single ring with alternating double and single bonds.
To designate an annulene, use brackets to denote the number of atoms in the ring and then add the word annulene. Therefore, benzene is [6]-annulene. Both [14]-annulene and [18]-annulene are aromatic compounds due to their cyclic, planar, and completely conjugated structure, as per Hückel’s rule.
Three-member ring compound
Cyclopropene, despite its cyclic and planar structure, does not have continuous electron delocalization. Thus, aliphatic or non-aromatic.
Cyclopropenyl cation’s positive charge creates an empty p orbital in conjugation with the double bond, resulting in continual electron delocalization. The double bond contains two electrons due to delocalization. As a result, it has aromatic properties.
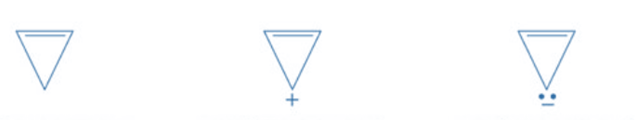
Cyclopropenyl anion’s negative charge causes two electrons in the p orbital to conjugate with the double bond, resulting in continuous electron delocalization. There are two electrons in the double bond and two electrons in the p orbital that contribute to continual delocalization. There are a total of four electrons. Thus, it is anti-aromatic.
Five-member ring compound
Despite its cyclic and planar structure, cyclopentadiene does not have continuously delocalized electrons. So this compound is non-aromatic.
The positive charge of a cyclopentadienyl cation creates an unoccupied p orbital in conjugation with two double bonds, resulting in continuous electron delocalization. Each double bond has two electrons due to delocalization. Hence, It has a total of four electrons. So, it is anti-aromatic.

A negative charge on cyclopentadienyl anion causes two electrons in the p orbital to conjugate with the double bond, resulting in continuous electron delocalization. There are 2 electrons in the double bond and 2 electrons in the p orbital, representing the negative charge involved in continual delocalization. It possesses a total of six electrons. Thus, it meets the criteria to be aromatic.
Six-member ring compound
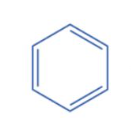
Benzene meets the first criteria due to its cyclic, planar structure and three conjugated double bonds, resulting in continuous electron delocalization. There are a total of six electrons involved in continuous delocalization, with 2 electrons in each of the three double bonds. Thus, it satisfies the second requirement and is aromatic.
Aromatic Compounds With More Than One Ring
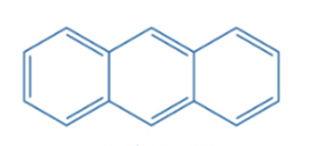
Hückel’s rule for determining aromaticity is only applicable to monocyclic systems, however, several aromatic compounds with multiple benzene rings linked together are also known. Polycyclic aromatic hydrocarbons (PAHs) are formed when two or more six-membered rings with alternating double and single bonds combine. Combining two benzene rings results in naphthalene. Three rings can be joined in two ways, resulting in anthracene.
Anthracene is classified as polycyclic aromatic hydrocarbons. Appreciating the aromaticity of such compounds necessitates a unique perspective and theoretical consideration. Clar’s aromatic π-sextet rule, developed by Eric Clar, provides the most comprehensive explanation of polycyclic aromatic hydrocarbons.
Clar’s rule is an empirical rule that describes the relationship between a molecule’s chemical stability and aromaticity. Erich Clar, an Austrian organic chemist, first used the term in his 1972 book The Aromatic Sextet. According to the rule, the resonance structure with the most aromatic π-sextets, or benzene-like moieties, is the most essential in characterizing the features of a polycyclic aromatic hydrocarbon.
Heterocyclic aromatic compounds
Aromatic heterocycles can include oxygen, nitrogen, or sulfur atoms with one or more lone pairs of electrons. When dealing with heteroatoms, it’s important to identify if the lone pair is localized on the atom or part of the delocalized system. for example:
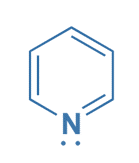
Pyridine
Pyridine is cyclic, planar, and completely conjugated, with three single and double bonds alternating around the ring. Pyridine contains six π electrons, two from each π bond, which satisfies Hückel’s rule and makes it aromatic. Pyridine’s nitrogen atom contains a nonbonded electron pair that is localized on the N atom and does not contribute to the aromatic ring’s delocalized electron system.
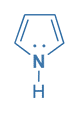
Pyrrole
In the case of pyrrole, the nitrogen atom’s lone pairs of electrons are partially delocalized into the ring, resulting in a 4n + 2 aromatic system. Therefore Pyrole is a cyclic, planar, fully conjugated, aromatic compound with 4n + 2 It electrons.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Sthapit, M. K., Pradhananga, R. R., Bajracharya, K. B., (2014). Foundations of chemistry. Taleju Prakashan.
- Arun Bahl, B.S. Bahl and G.D. Tuli. (1999). Study Guide and Solutions Manual For : Essentials of Physical Chemistry (1). New delhi: S. CHAND.
- https://www.vedantu.com/chemistry/huckels-rule
- https://www.chemistrysteps.com/identify-aromatic-antiaromatic-or-nonaromatic-compounds/
