Benzene, an aromatic hydrocarbon, having the chemical formula C6H6, is one of the most significant organic molecules. It is the primary component of all aromatic compounds.
At room temperature, It is a colorless or light-yellow liquid compound. It is largely utilized as a solvent in the pharmaceutical and chemical fields, as a starting material and intermediate in the production of several compounds, and as a component of gasoline. The term “benzene” is derived from “gum benzoin” (benzoin resin), which is an aromatic resin. It is a naturally occurring component of crude oil, which is the primary source of benzene today. Volcanic gas emissions and forest fires are examples of other natural sources.
Interesting Science Videos
Discovery of benzene
The term “benzene” is derived from the fragrant resin “gum benzoin,” which is produced in Southeast Asia. It has been known to European perfumers and chemists since the early 16th century. Sublimating benzoin created an acidic compound known as “flowers of benzoin” or benzoic acid. As a result, the hydrocarbon formed by benzoic acid became known as benzene, benzol, or benzene.
In 1825, Michael Faraday discovered benzene in compressed illuminating lighting gas. In 1834, Eilhardt Mitscherlich, a German chemist, heated benzoic acid with lime to produce benzene. Later, in 1845, German scientist A.W. von Hofmann isolated benzene from coal tar.
Structure of benzene
It is the most basic aromatic hydrocarbon, made up of six carbon atoms linked together by single and double bonds. Each carbon atom is linked to a single hydrogen atom. A resonance structure is formed by six conjugate carbon atoms and p-orbital delocalization resulting from sp2 hybridization.
All of its carbon-carbon bonds are identical intermediates that are halfway between the lengths of a single and a double bond. In benzene, each carbon atom is connected to two other carbon atoms and just one hydrogen atom, with a bond angle of 120° between each connection. As a result, it is a trigonal planar molecule.
The fourth outer-shell electron of carbon is in a pi orbital, while the bonded three are in sigma orbitals. Sigma orbitals connect atoms, whereas pi orbitals extend above and below the atom. In benzene, all of the carbon atoms’ pi orbitals overlap, resulting in a connected region that stretches above and below the molecule.
Electrons can move anywhere inside this overlapping zone, indicating that they are delocalized. The term “delocalized pi system” refers to the entire structure. As a result of the delocalization of pi- electrons, all of its C-C bonds are identical intermediates.
Characteristics of benzene
- A hexagonal planar molecule composed of six carbon atoms.
- The chemical formula is derived from the fact that each carbon atom has a single connection with a hydrogen atom. C6H6
- Carbon atoms alternately share single and double bonds, resulting in a bond length that falls approximately between single and double bonds.
- Delocalized pi electrons are present above and below the plane of the ring. it cannot participate in addition reactions because adding compounds could break the ring, rendering it unstable.
Aromaticity of benzene
Since the C-C bonds generated in the ring are not exactly single or double, but rather of intermediate length, it is an aromatic chemical. Based on Huckel’s rule aromatic chemicals are classified into two types: benzenoids (those with a benzene ring) and non-benzenoids (those without a benzene ring). According to the Huckel rule, an aromatic ring should contain the following properties:
- It must be planar.
- There must be a presence of complete electron delocalization in the ring
- The presence of (4n + 2) electrons in a ring, where n is an integer (n = 0, 1, 2,…)
Preparation of benzene
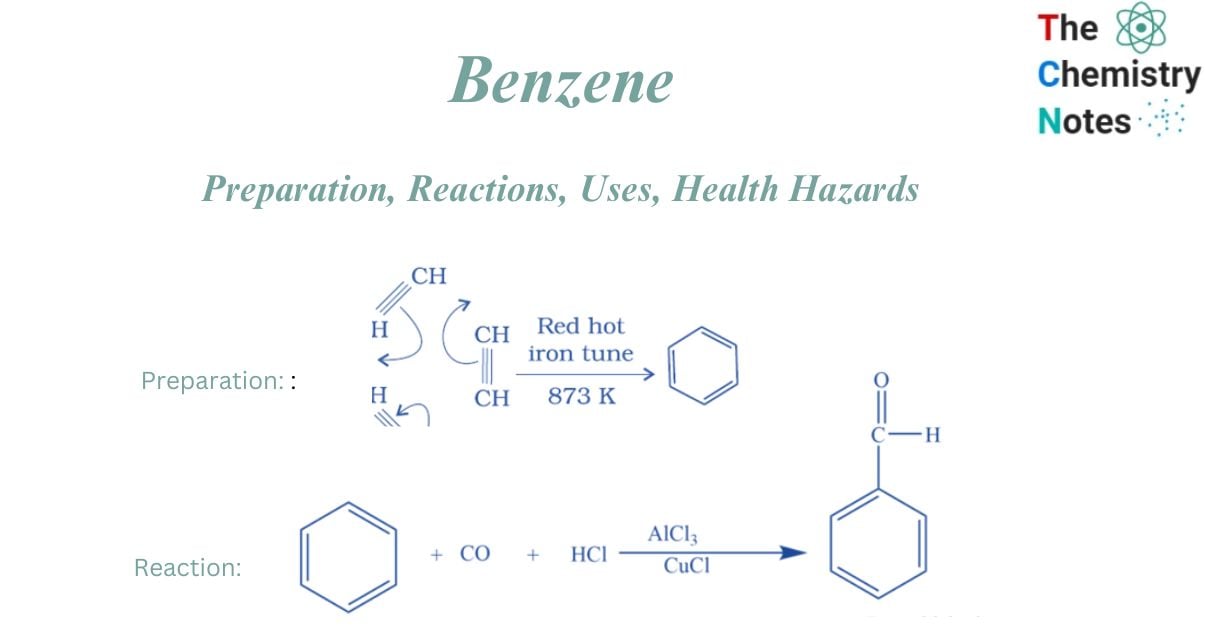
Cyclic polymerization of ethyne
It can be prepared from cyclic polymerization of ethyne. This is accomplished by passing Ethyne through a red hot tube at a temperature of 873K, where it transforms into Benzene.
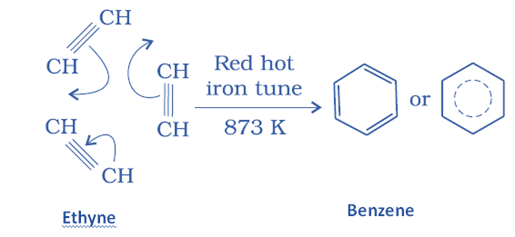
Aliphatic hydrocarbon aromatization or hydroforming
This is the conversion of aliphatic hydrocarbons to aromatic hydrocarbons. This process is sometimes referred to as hydroforming or catalytic reforming.
Aromatic hydrocarbon is formed when an alkane with six or more carbon atoms is heated forcefully (775 K) under pressure in the presence of a platinum catalyst.
This method entails cyclization, isomerization, and dehydrogenation. The result of this procedure has the same number of carbon atoms as the aliphatic starting ingredients. Because unsaturated hydrocarbons are better fuels, aromatization raises the octane number of petrol from 40 to 95.
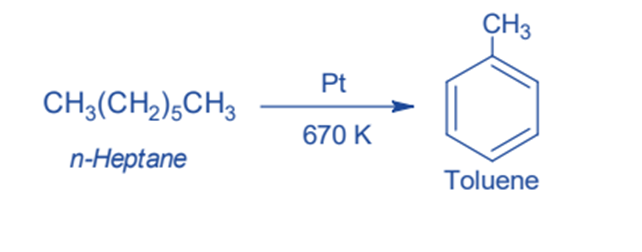
Decarboxylation of sodium benzoate
This is the laboratory procedure for obtaining Benzene from Sodium benzoate. When Sodium benzoate and Soda-lime (Sodium Hydroxide and Calcium Oxide) are heated they produce benzene by decarboxylation. In this method, sodium carbonate is obtained as a byproduct.
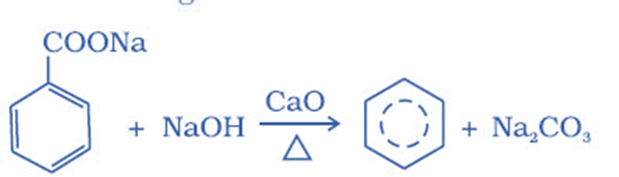
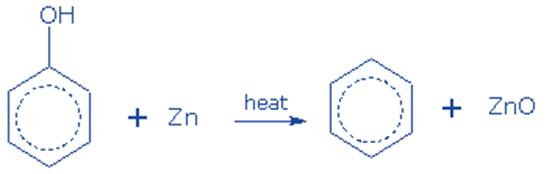
Reduction of phenol
It can be produced by reducing phenol. It is generated when phenol vapors are passed over heated zinc dust. This reaction occurs in the presence of strong reducing chemicals, such as zinc, and under intense heating.
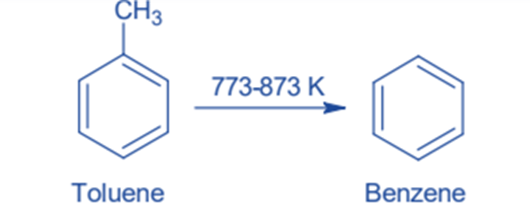
Toluene Hydrodealkylation
Dealkylation can convert toluene to benzene. Toluene and hydrogen are heated at high temperatures (773-873 k) and pressures of 40—60 atm in the presence of a chromium, molybdenum, or platinum oxide catalyst in this reaction. At identical reaction conditions, much higher temperatures are sometimes employed instead of a catalyst. Toluene is dealkylated to benzene and methane under these circumstances. This process produces a high yield.
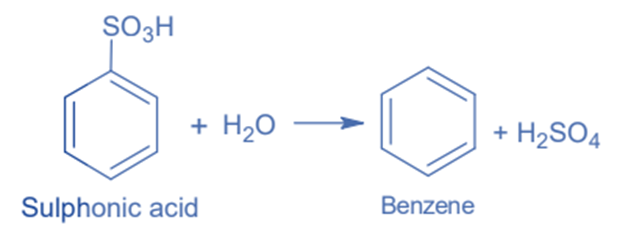
Hydrolysis of sulphonic acid
Sulphonic acid hydrolysis can be used for its synthesis. Sulphonic acid is exposed to superheated steam in this reaction, resulting in the synthesis of benzene.
Properties of benzene
- It is immiscible in water and cannot create a homogenous mixture with it. It is, however, soluble in organic solvents.
- It is a liquid, colorless aromatic compound with an aromatic smell.
- It is highly flammable and produces a sooty flame when burned.
- It exhibits resonance and can exist in several forms depending on the position of the double bond, making it exceedingly stable.
- It is lighter than water, with a density of 0.87g/ cm3.
- Its boiling point is 80.5 oC, and the melting point is 5.5 oC.
- With halogen acids and hypochlorous acids, it does not give any additional products.
- It is resistant to oxidation by KMnO4 (i.e., no decolorization) and does not decolorize bromine solution. However, it exhibits some additional reactions under certain conditions.
- It behaves in many ways like a saturated hydrocarbon because it undergoes substitution processes, in which hydrogen atoms are replaced by other atoms or groups (it is more reactive than alkanes).
- It is unaffected by alkalis such as NaOH.
- It exhibits addition reactions, electrophilic aromatic substitution reactions, and oxidation reactions in general.
Reactions of benzene
Reduction
Cyclohexane is produced by hydrogenating benzene at higher temperatures and pressures.

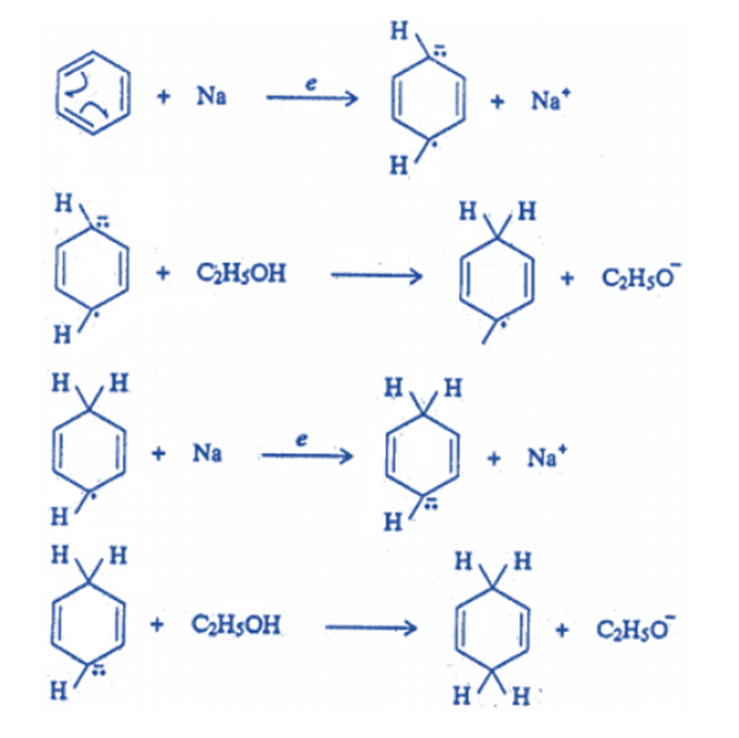
it is not reduced by metal and acid or sodium in ethanol, but it is reduced by sodium in liquid ammonia in the presence of ethanol (Birch reduction) to yield 1, 4-dihydro benzene (cyclohexane-1, 4-diene). This reaction has also been linked to a free radical mechanism.
Nitration
The nitronium ion, NO2+, which serves as our electrophile, is used to nitrate benzene. It is formed by the reaction of concentrated sulfuric and nitric acids (H2SO4 and HNO3). Because sulfuric acid is a stronger acid than nitric acid, nitric acid must serve as a base, accepting a proton given up by sulfuric acid. The reaction produces H2NO3+ and the bisulfate ion (HSO4), and the H2NO3+ degrades into water and a nitronium ion. The overall reaction is as follows:

Nitronium ion is an electrophile, which means it has a vacant electron orbital and a positive or partially positive charge. The nitronium ion combines with benzene because it is attracted to the ring of delocalization, which has a high electron density, and it takes the place of one of the hydrogen atoms. To prevent volatile components from escaping, it is heated at 50 °C with strong sulfuric and nitric acids using reflux. This results in the formation of nitrobenzene (C6H5NO2).
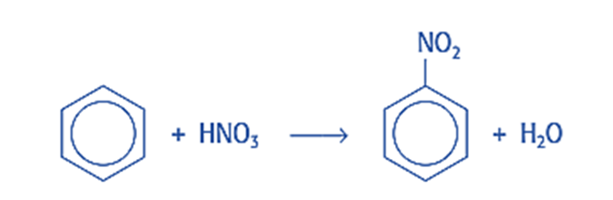
Halogenation reaction
In the presence of Lewis acid, it interacts with halogens to generate aryl halides. This process is known as halogenation of benzene.

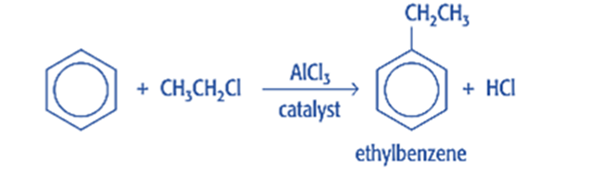
Friedel craft alkylation
Friedel-Crafts alkylation is the substitution of an aromatic proton by an alkyl group. This is accomplished through an electrophilic attack on the aromatic ring using a carbocation. The Friedel-Crafts alkylation reaction uses alkyl halides as reactants to produce alkylbenzenes.
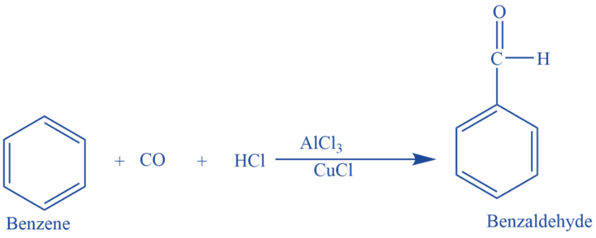
Gattermann -Koch aldehyde synthesis
In the presence of anhydrous aluminum chloride and traces of copper (I) chloride, it reacts with carbon monoxide and hydrochloric acid to produce benzaldehyde. This reaction is known as Gattermann -Koch reaction. Here copper (I) chloride acts as a co-catalyst.
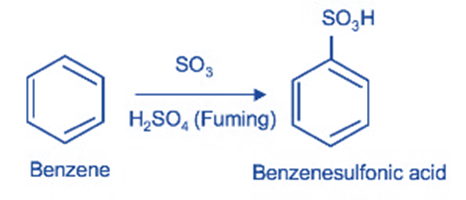
Sulphonation
When it combines with sulfur trioxide (SO3), it undergoes sulfonation to produce benzene sulphonic acid. Sulfuric acid functions as a catalyst in this process. A catalyst is a species that improves the reactivity of reagents in a chemical reaction.
Applications of benzene
- It is employed in the synthesis of phenol. It is also used to make aniline, which is used in dyes, and dodecylbenzene, which is used in detergents.
- It was originally used to degrease metal.
- It is utilized in the production of nylon fibers.
- Its primary application is in the production of other compounds such as ethylbenzene, cyclohexane, cumene, nitrobenzene, alkylbenzene, and so on.
- Many compounds, including fats, oils, resin, rubber, sulfur, iodine, and phosphorus, employ it as a liquid solvent.
- It is used to create lubricants, dyes, detergents, medicines, explosives, and other industrial compounds.
Health hazards of benzene
- Long-term exposure to it can primarily impact our blood. As a result, it causes anemia by reducing the number of red blood cells in our bodies.
- A high level causes irregular menstruation cycles and shrinks women’s ovaries.
- It also has an impact on the development of fetuses in pregnant women and male fertility.
- It possesses cancer-causing characteristics. As a result, long-term high amounts of benzene exposure induce cancer and leukemia in humans.
- Acute exposure causes toxic effects on the central nervous system; however, to assess the chronic effects, the myelotoxic, chromosomal damaging, and leukemogenic effects of benzene must be considered.
References
- https://byjus.com/chemistry/benzene/.
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Sthapit, M. K., Pradhananga, R. R., Bajracharya, K. B., (2014). Foundations of chemistry. Taleju Prakashan.
- Arun Bahl, B.S. Bahl and G.D. Tuli. (1999). Study Guide and Solutions Manual For : Essentials of Physical Chemistry (1). New delhi: S. CHAND.
- https://colapret.cm.utexas.edu/courses/Chapter%2022-benzos.pdf.
- https://www.egyankosh.ac.in/bitstream/1234
