Phenols are aromatic compounds with a hydroxyl group directly connected to an aromatic ring. They are hydroxy derivatives of benzene. The general formula of phenol is C6H6O.
Phenol was discovered by Runge (1834) in coal-tar and he named it carbolic acid (Carbo = coal, oleum = oil). It is also known as carbolic acid. Previously, phenol was used as carbolic soap. it is mildly acidic and corrosive to the respiratory tract and skin. It can cause chemical burns.
Interesting Science Videos
Classification of phenol
Common name system
Phenols are classified as monohydric, dihydric, or trihydric based on the number of hydroxyl groups attached to the benzene ring.
Monohydric phenols: They contain only one -OH group and are the most basic member of the series hydroxybenzene. They are also known as phenol, while others are referred to as substituted phenols. Cresols (ortho, para, and meta) are the three isomeric hydroxyl toluenes.
Dihydric phenols: Dihydric phenols consist of two -OH groups. They can also be ortho-, meta-, or para-derivatives. Catechol, resorcinol, and quinol are common examples of dihydric phenols.
Trihydric phenols: Trihydric phenols have three -OH groups. Pyrogallol, hydroxyquinol, and phloroglucinol are common examples of trihydric phenols.
IUPAC nomenclature system
A set of rules governs the IUPAC nomenclature of phenols. the rules are:
1. Identify the position of a hydroxyl group on the benzene ring.
2. To denote the number of similar hydroxyl groups attached to the benzene ring, benzene rings with more than one hydroxyl group are labeled with Greek numerical prefixes such as di, tri, and tetra. E.g., When another hydroxy group is present in the meta position to the first one, it is referred to as benzene 1,3 diol.
3. The positions of the other functional groups in substituted phenols are located in relation to the position of the hydroxyl group. For instance, if a methyl group is attached to carbon number three in relation to the hydroxy group, the compound is known as 3-Methyl phenol.
4. Depending upon the position of the substituted functional group with respect to the hydroxyl group, words such as ortho, para, and meta are used for the nomenclature of phenols.
| Structure | Common name | IUPAC name |
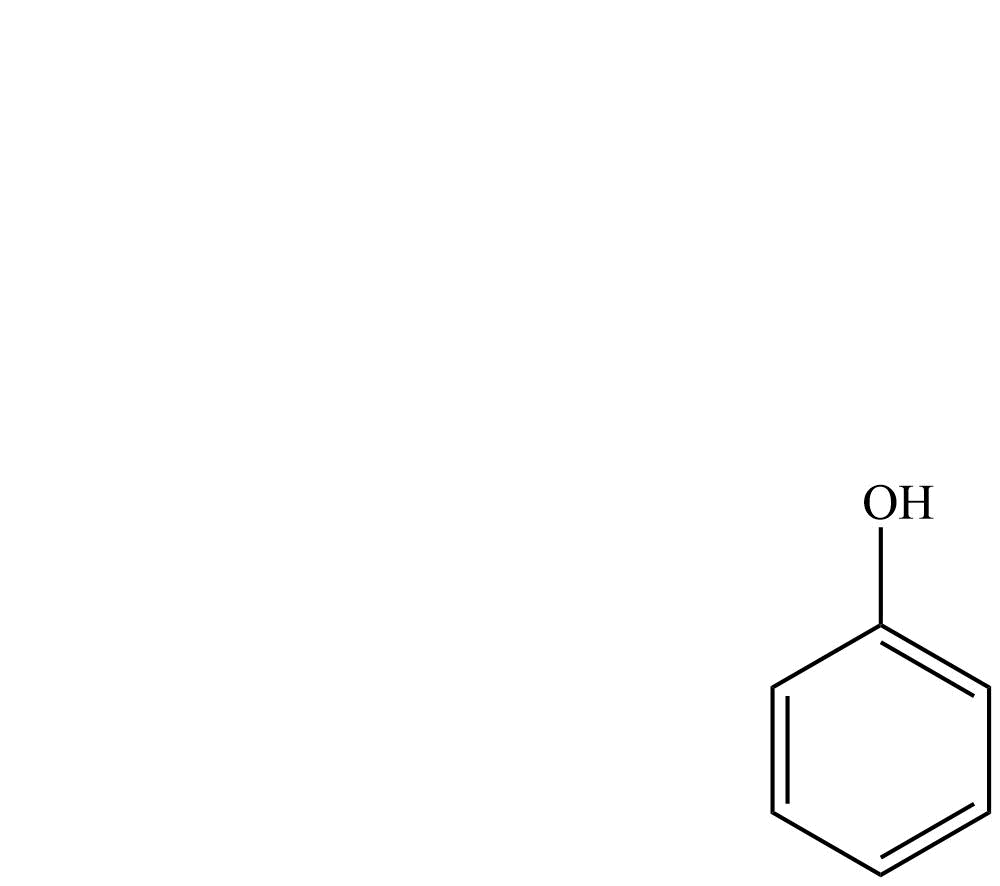 | Phenol | Benzenol |
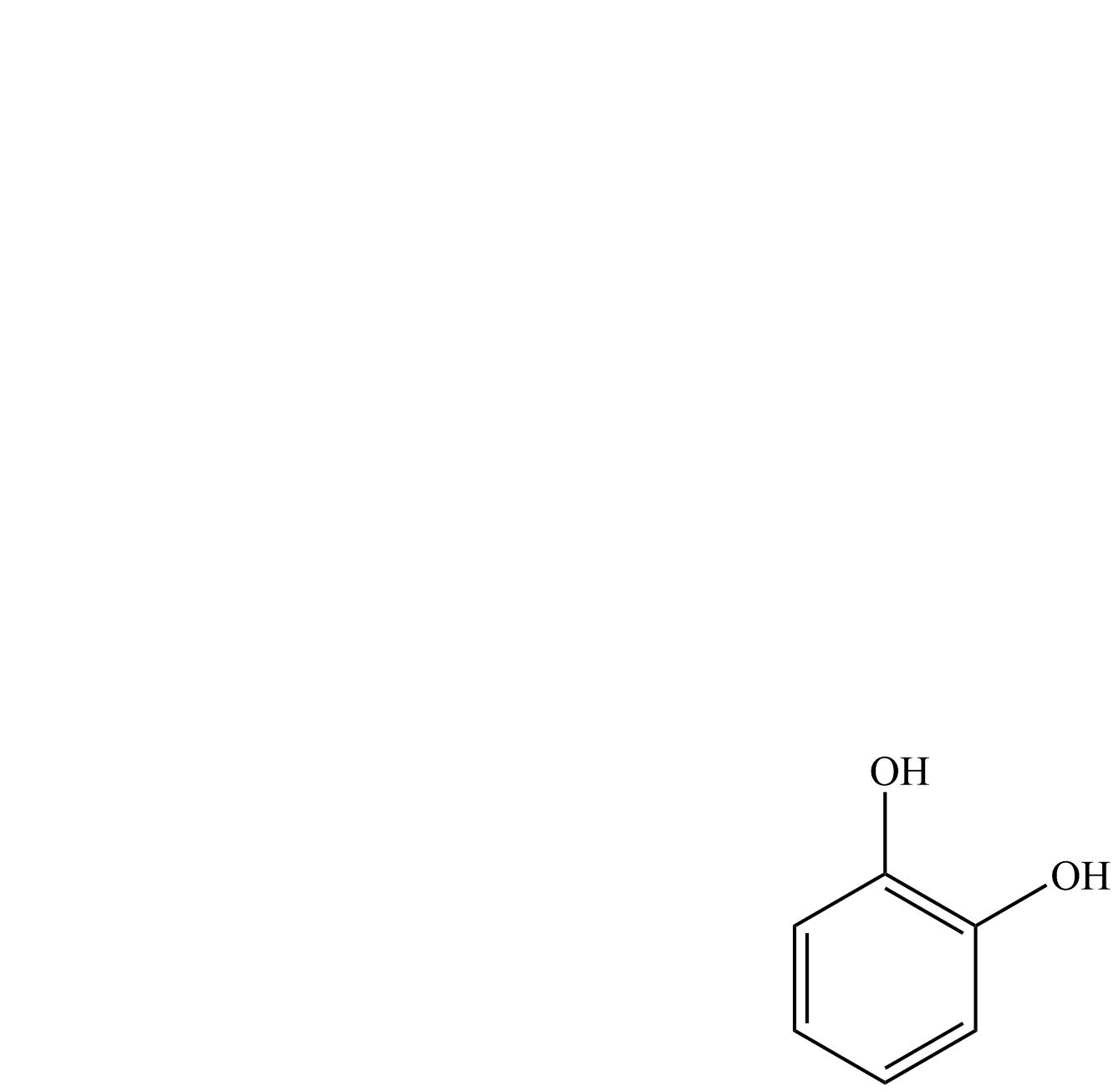 | Pyrocatechol | Benzene-1,2- diol |
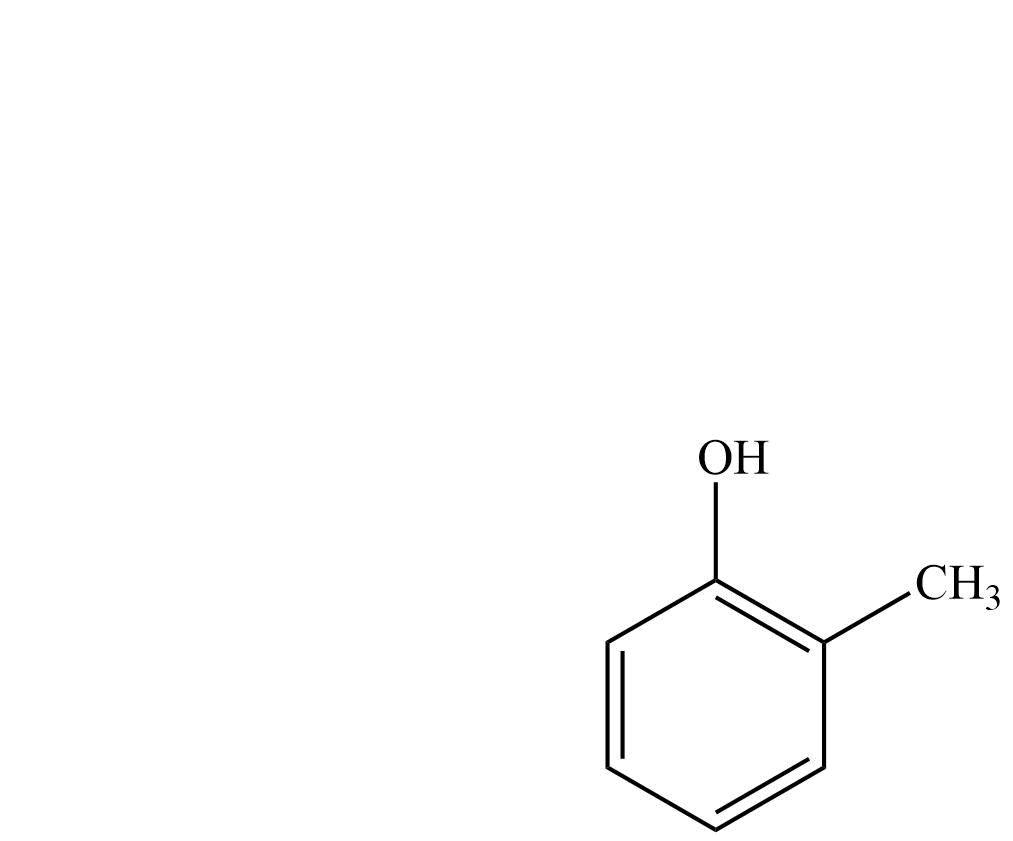 | o-Cresol | 2-Methylbenzenol |
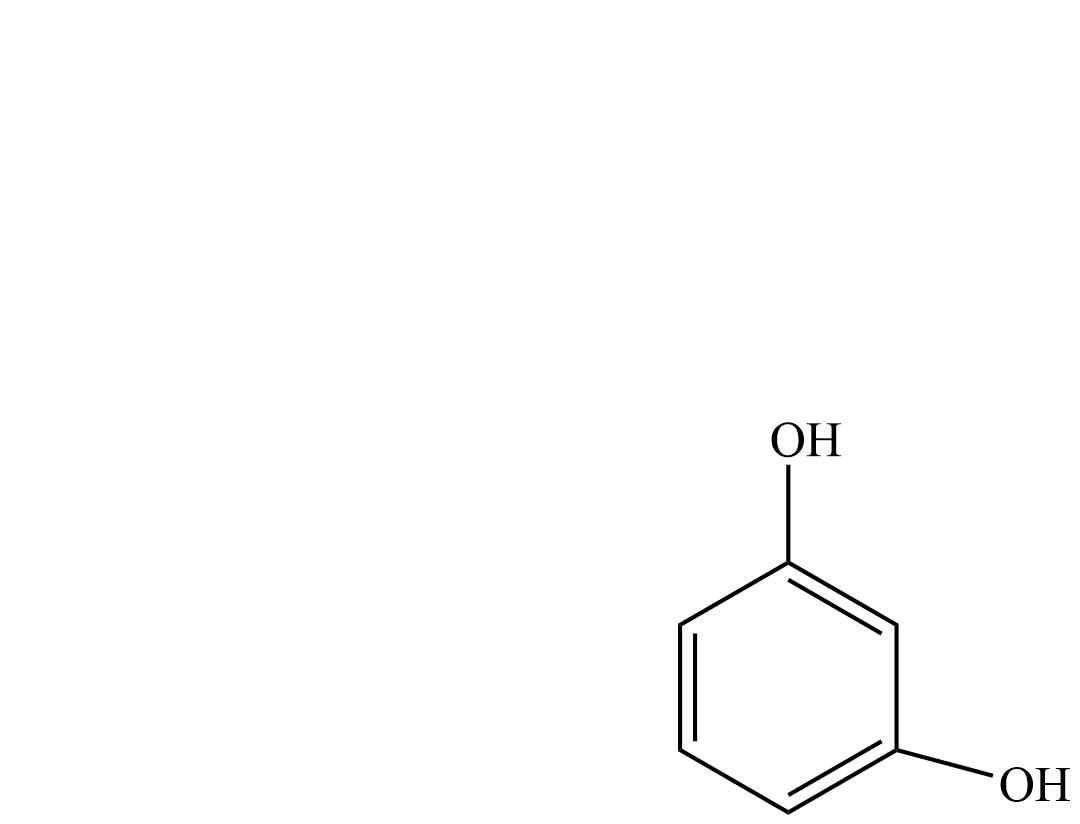 | Resorcinol | Benzene-1,3-diol |
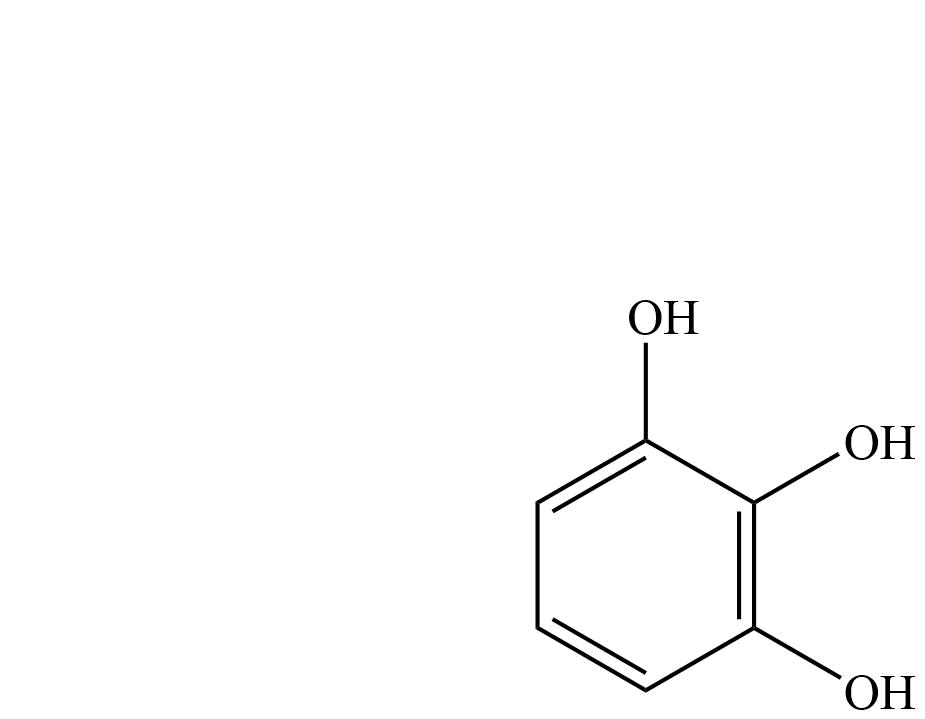 | Pyrogallol | Benzene-1,2,3-triol |
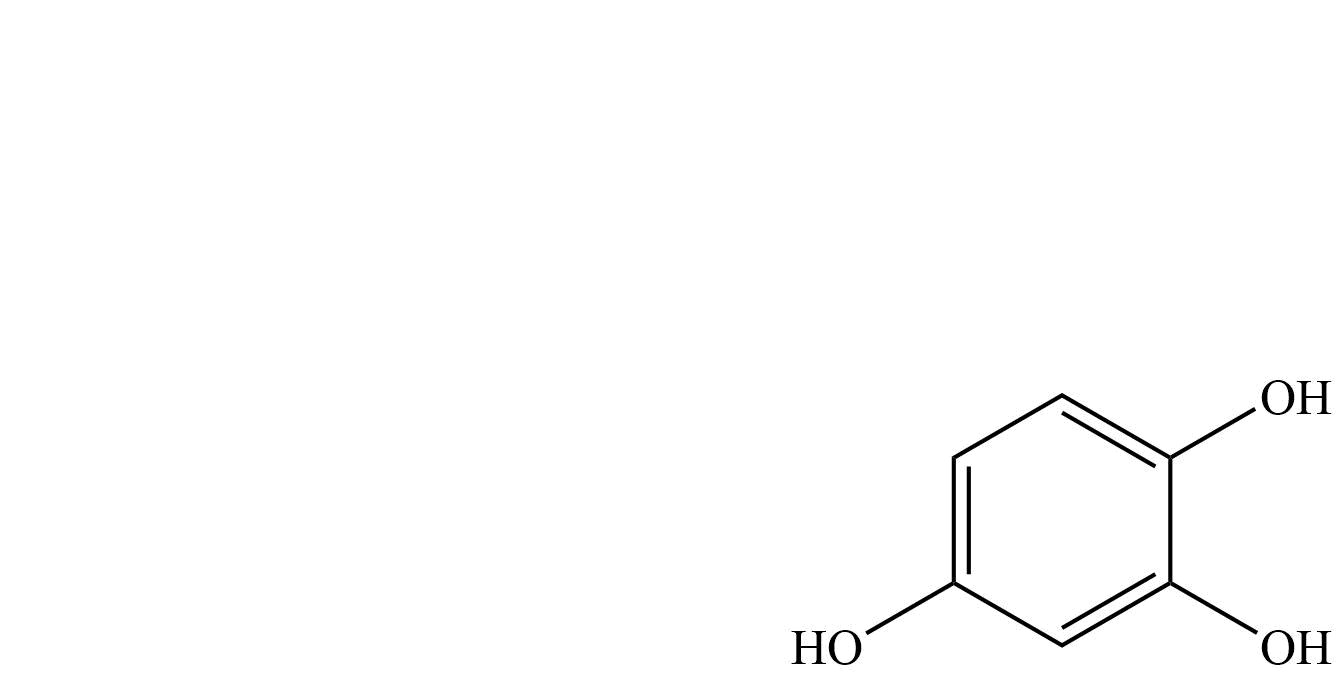 | Hydroxyquinol | Benzene-1,2,4-triol |
Preparation of phenol
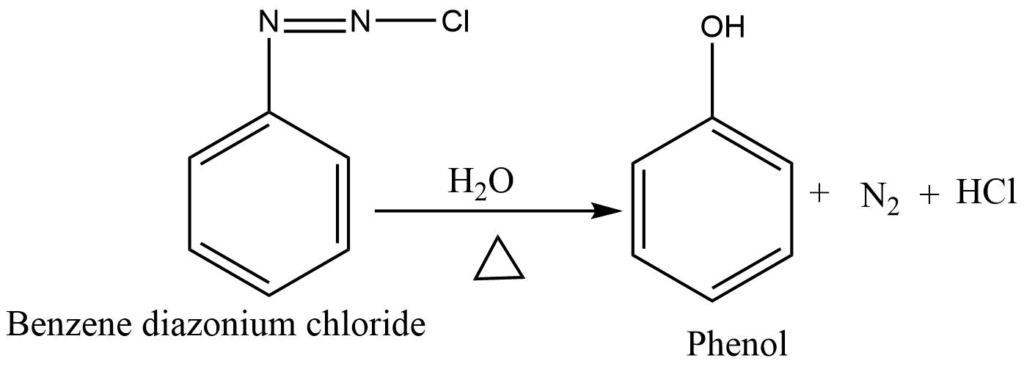
From diazonium salt
Diazonium salts are hydrolyzed to phenols in the presence of dilute acids.
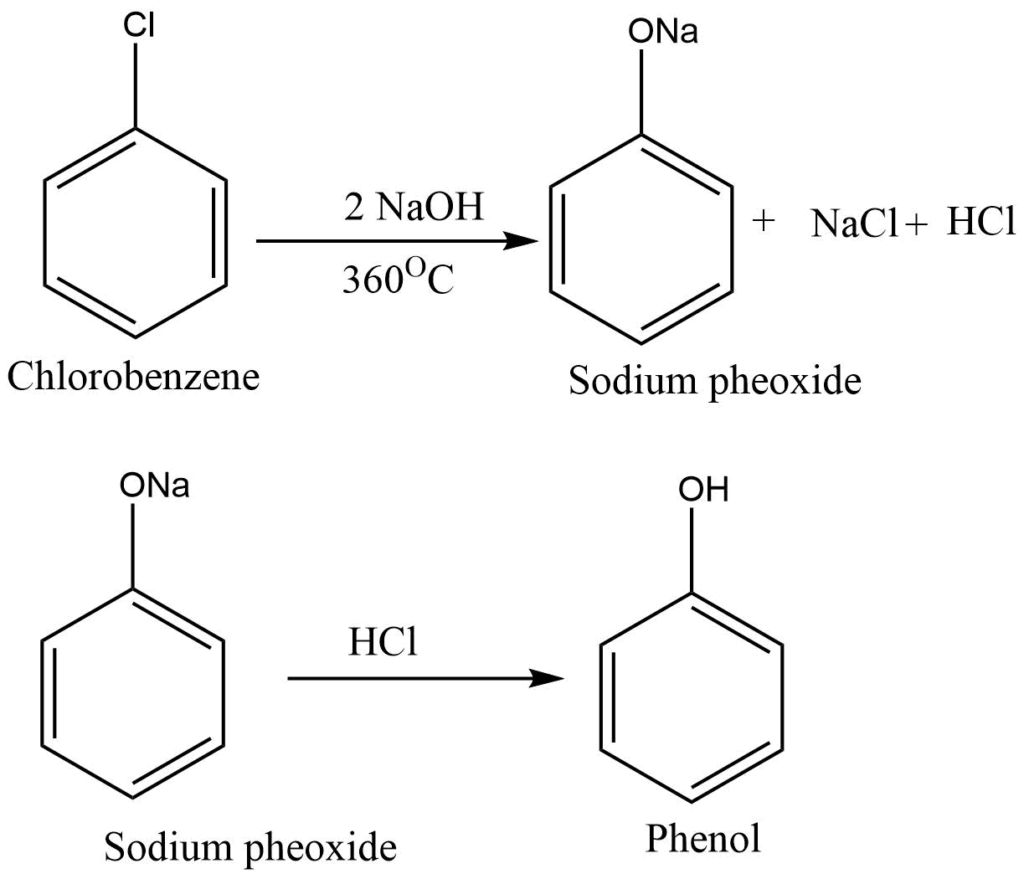
By Dow’s process (from chlorobenzene)
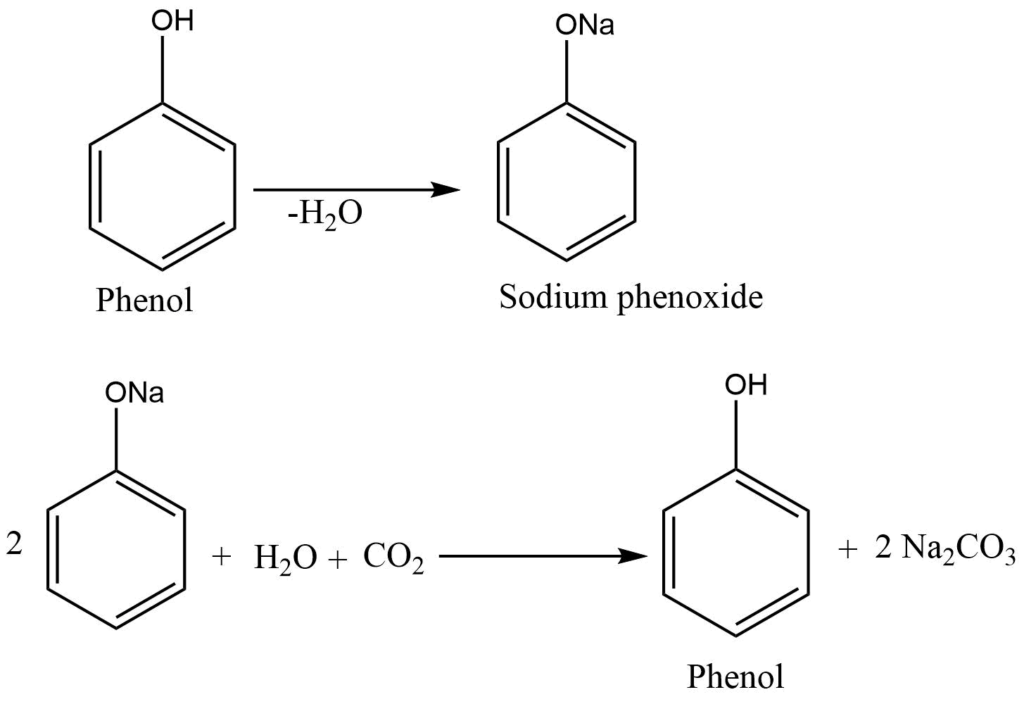
Chlorobenzene, obtained by the chlorination of benzene, is treated with sodium hydroxide to form sodium phenoxide. Sodium phenoxide, in reaction with dilute hydrochloric acid yields phenol.
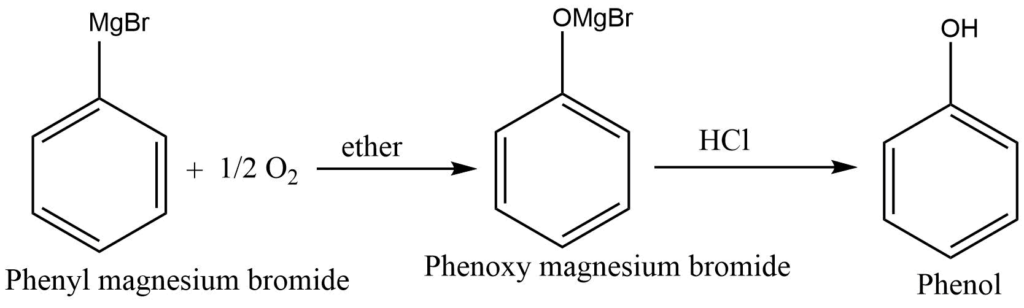
From Grignard reagent
The bubbling of oxygen through the ethereal solution of phenyl magnesium bromide produces an additional product that hydrolysis in an acidic medium produces phenol.
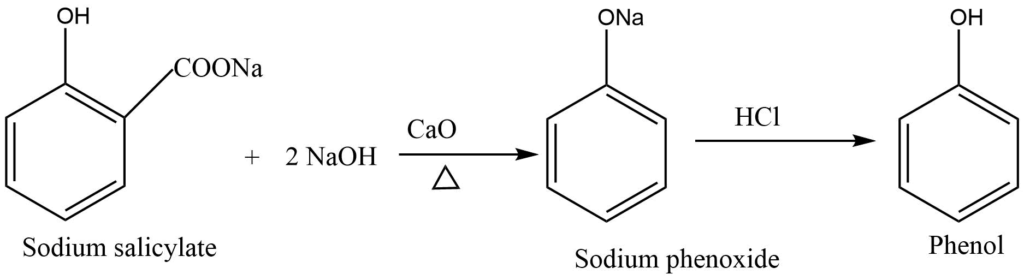
By sodium salicylate
On distillation with soda lime, sodium salicylate undergoes decarboxylation to form sodium phenoxide, which on acidification, yields phenol.
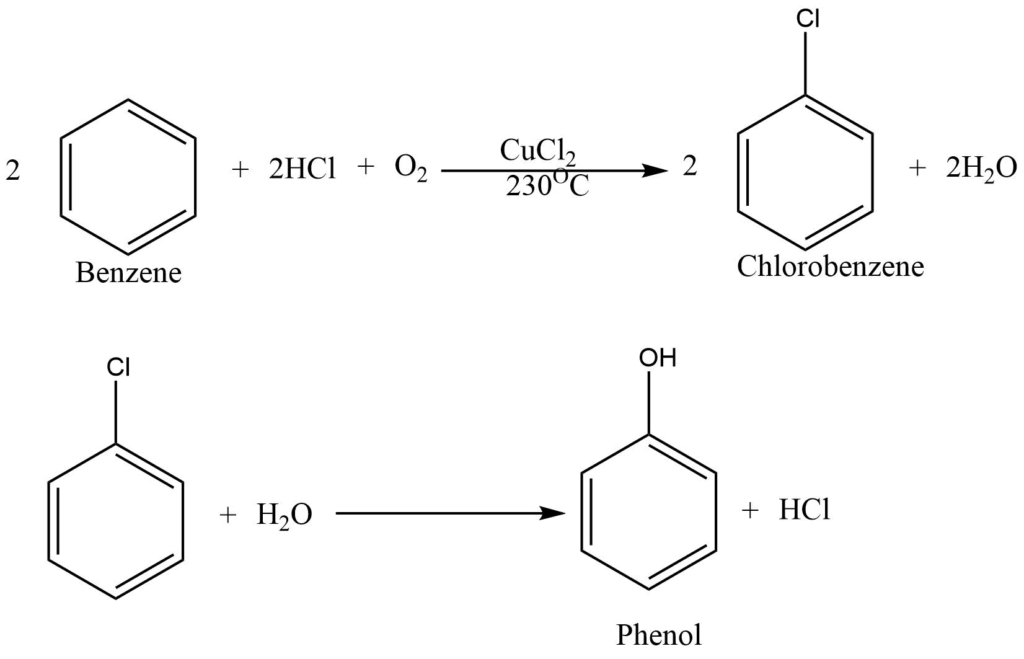
From benzene (Raschig process)
Chlorobenzene, obtained from the catalytic oxidation of a benzene and hydrogen chloride, is hydrolyzed by its mixture with steam over a Si catalyst at 500oc to produce phenol.
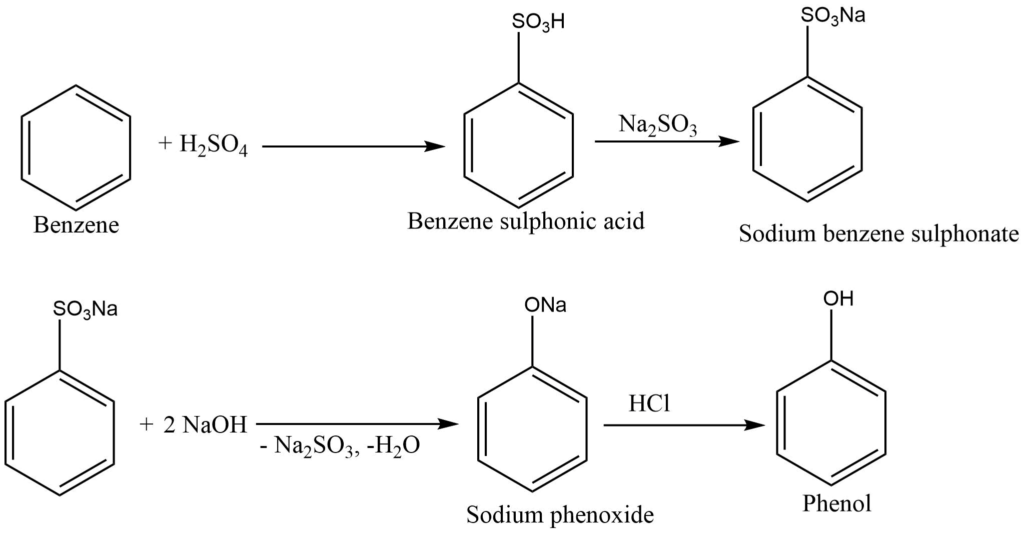
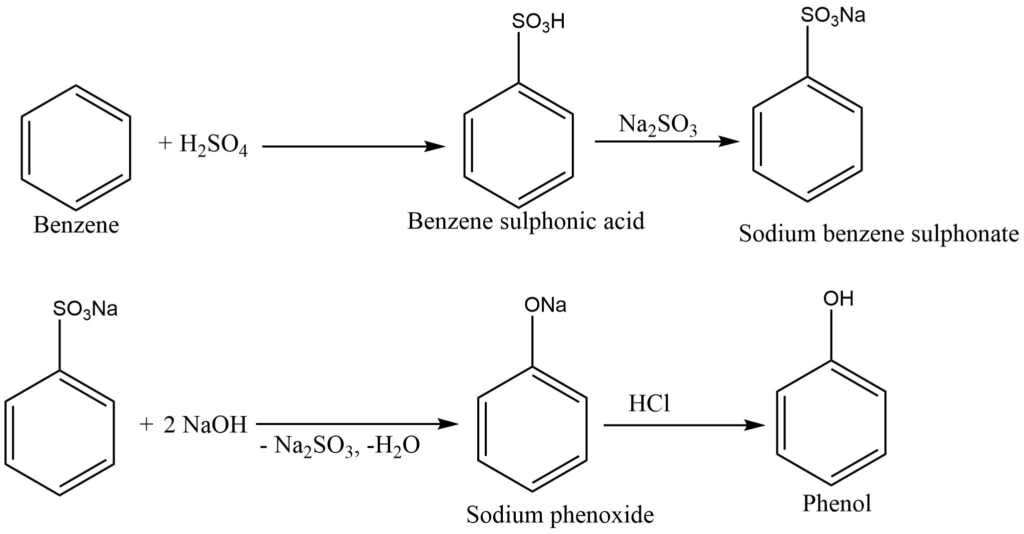
By sulphonation process
Benzene, in reaction with concentrated sulphuric acid, gives benzene sulphonic acid, which on neutralization with sodium hydroxide or sodium sulfite, produces sodium sulphonate. Sodium sulphonate is fused with caustic soda to give sodium phenoxide. This product, on hydrolysis and then acidification with SO2 or HCl, produces phenol.
By direct oxidation of benzene
Benzene with air is passed over vanadium phenoxide at 500-600 oC to get phenol.
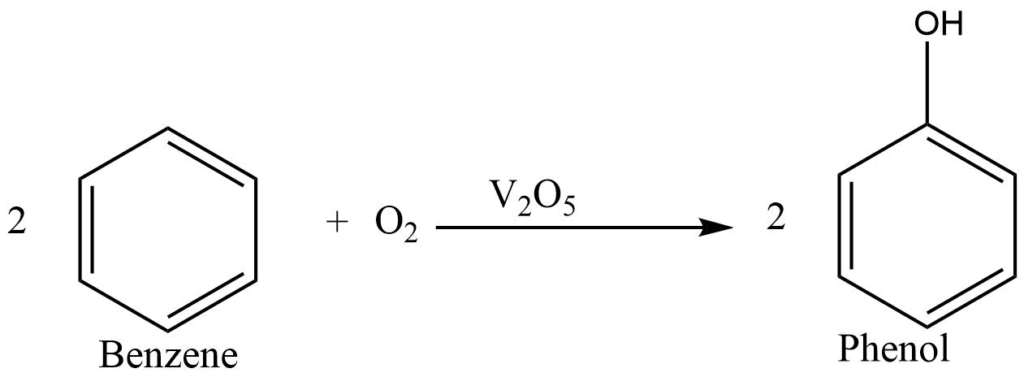
From coal tar
The middle oil fraction (170o– 230o C) of coal tar contains phenol, cresols, and xylols along with naphthalene. The naphthalene crystallizes on cooling. The resulting mixture on ignition with caustic soda solution phenol and cresol produces their sodium salt. The sodium salts so obtained are then treated with carbon dioxide. The phenol is removed by fractional distillation.
Physical properties of phenol
- It is a white hygroscopic crystalline solid with a strong phenolic odour.
- They have higher melting and boiling point than arenes and haloarene of comparable molecular mass. The phenol has m.p 43oC b.p. 182 oC.
- They are only slightly (sparingly) soluble in water, even though they can form intermolecular H-bonds with water molecules. The hydrophobic nature of the benzene ring contributes to the low solubility.
- On exposure to light and air, it develops slight pink color due the partial oxidation.
- It is very poisonous and can cause painful blisters in contact with skin.
Chemical properties of phenol
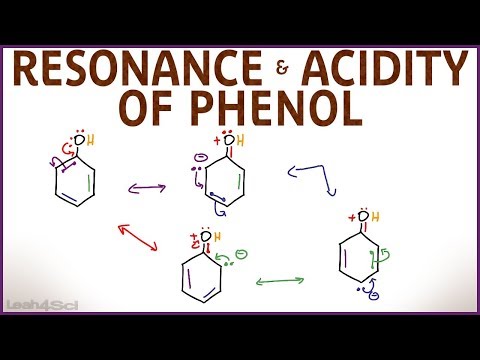
The reactions of phenol are those of the phenyl and -OH groups. The phenolic -OH group exhibits the typical replacement reactions of the aliphatic -OH group but differs from the alcoholic -OH as it has a weakly acidic character.
The acidic nature of phenol is due to the following:
1. The tendency of the oxygen atom to share its lone pair of electrons with the benzene ring gives rise to resonance.
2. The residual phenolate ion, due to proton removal, shows stability due to resonance.
Reaction due to -OH group
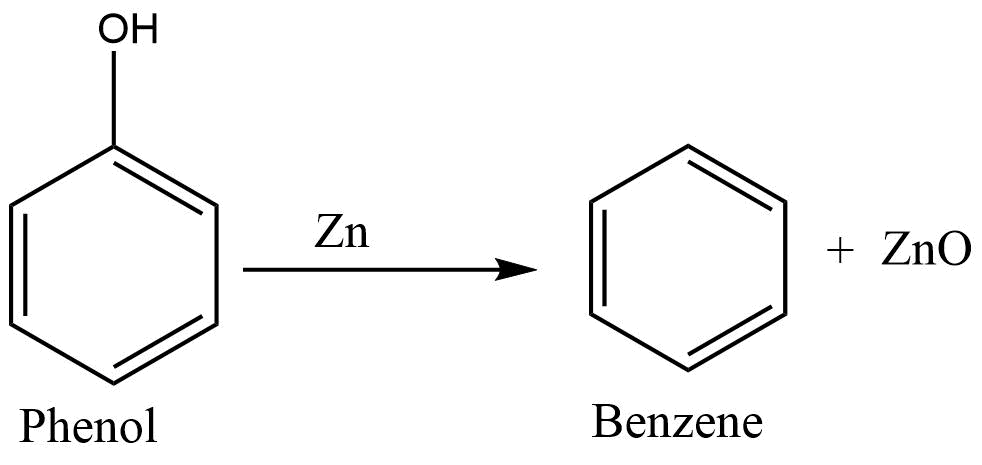
Reduction of phenol
Phenol, on heating with the Zn dust, undergoes reduction to form benzene.
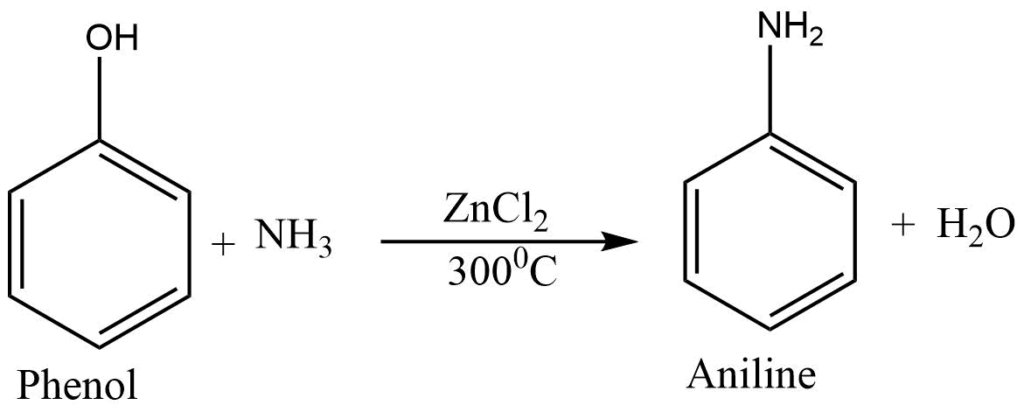
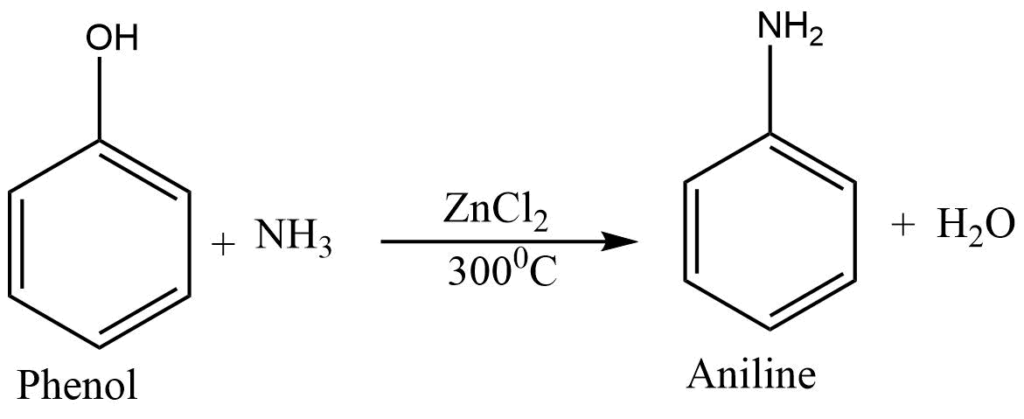
Reaction with ammonia
At high temperatures, phenol reacts with ammonia in the presence of anhydrous ZnCl2 to produce aniline.
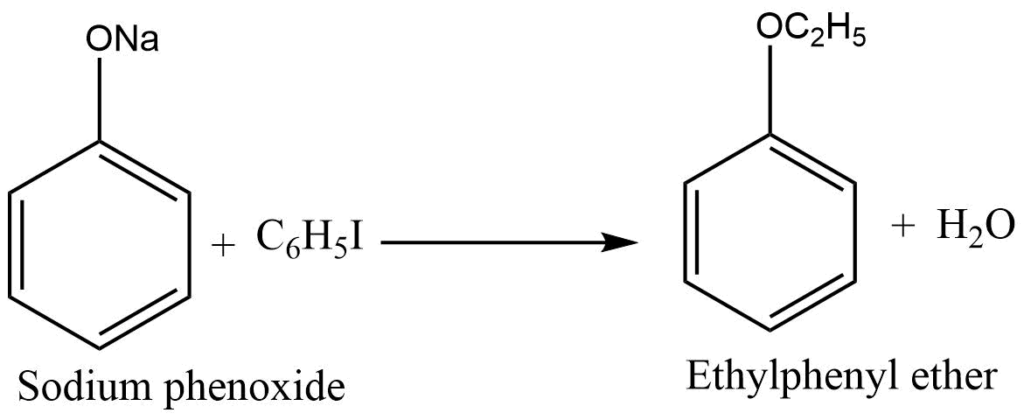
Ether formation (Williamson’s synthesis)
Sodium phenoxide reacts with an alkyl halide to form ether.
Ester formation
Phenol reacts with an acyl halide, aryl halide, or acid anhydride in an alkali solution to form phenyl esters.
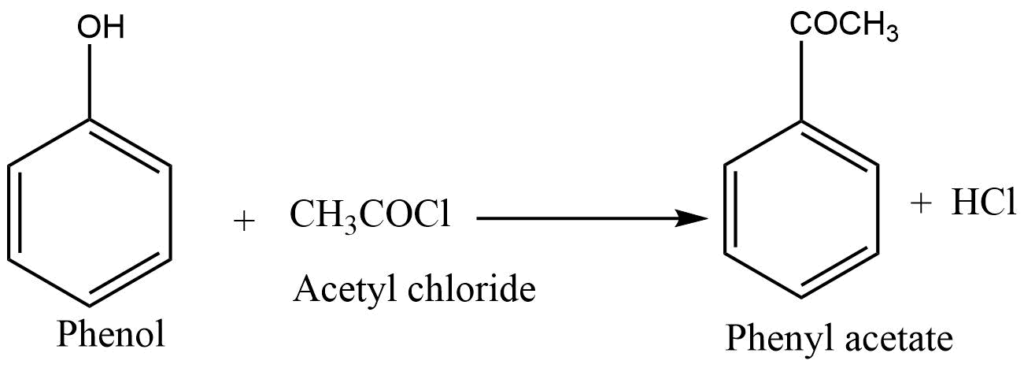
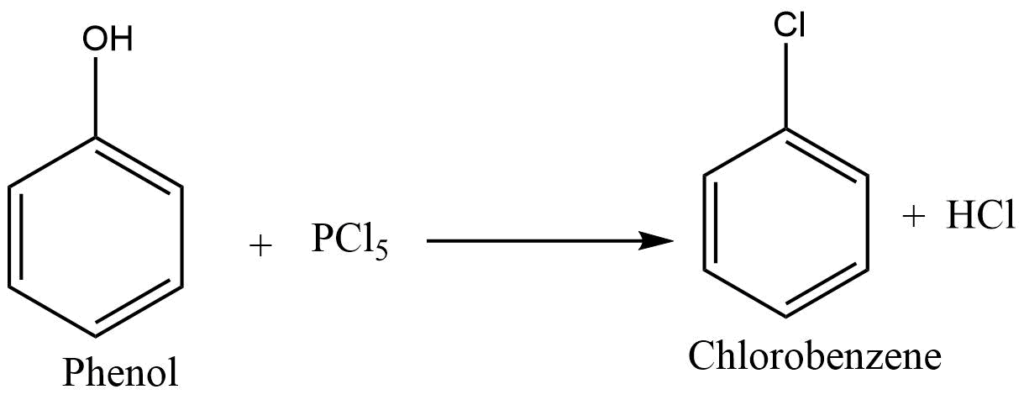
Reaction with PCl5
Phosphorous pentachloride reacts with phenol to give chlorobenzene.
Reaction with benzoyl chloride
Benzoyl chloride reacts with phenol in the presence of aqueous NaOH to form phenyl benzoate.
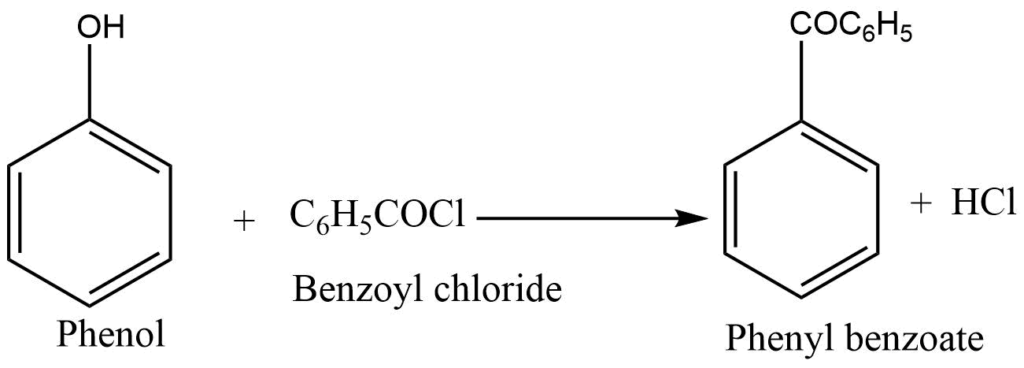
Reaction due to benzene ring
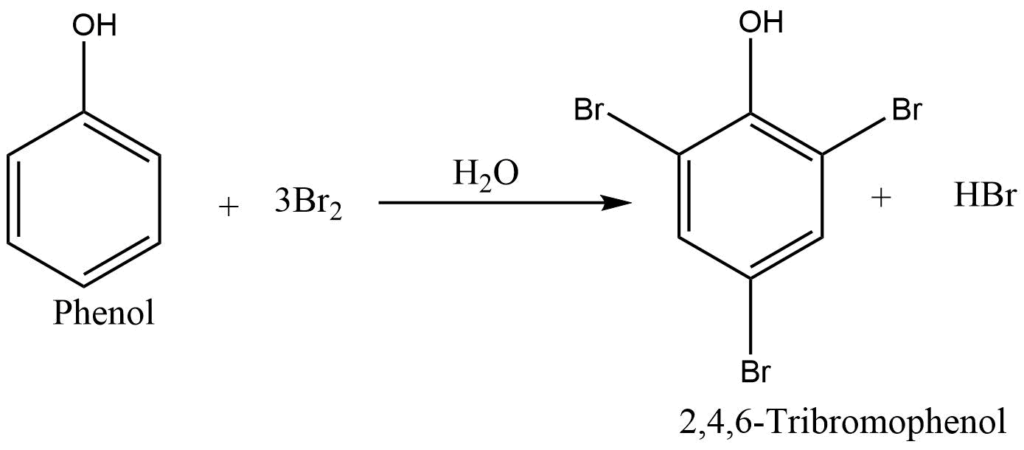
Electrophilic substitution reaction
a. Halogenation: When bromine water is added to phenol, a white precipitate of 2,4,6- tribromophenol is formed.
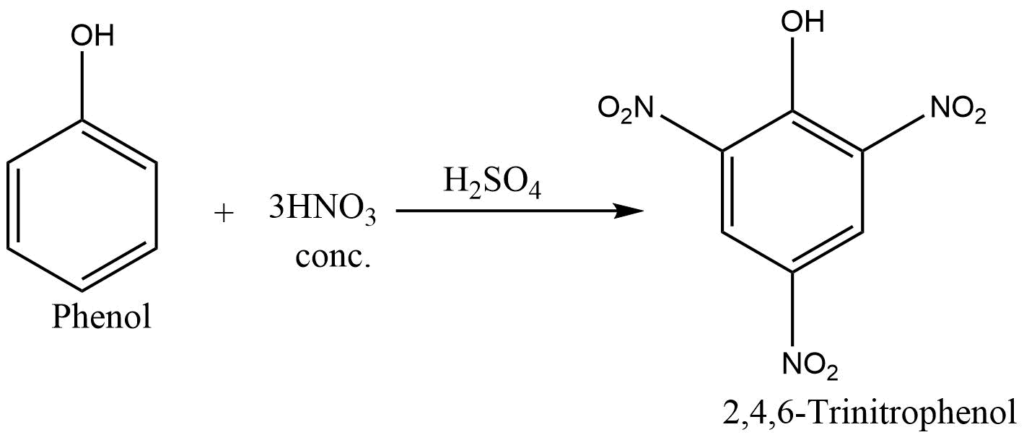
b. Nitration: Phenol reacts with conc. nitric acid in the presence of sulphuric acid to form 2,4,6-trinitrophenol (picric acid). But in the presence of dilute nitric acid, it gives a mixture of ortho and para nitrophenol.
c. Sulphonation: Reaction of phenol with conc. sulphuric acid gives a mixture of ortho and para phenol sulphonic acid.
Catalytic hydrogenation
Phenol reacts with hydrogen in the presence of a nickel catalyst at 150°C to from cyclohexanol.
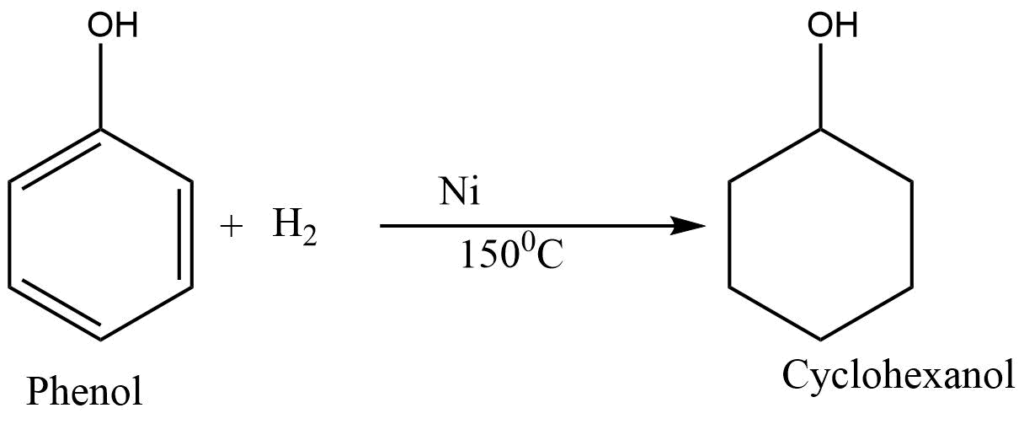
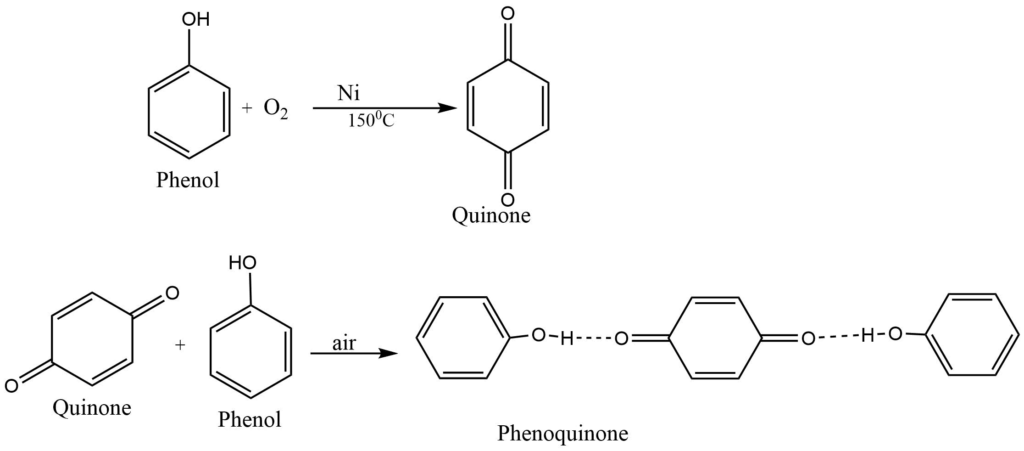
Oxidation
Exposure to air and light, phenol turns pink due to partial oxidation. It forms a complex mixture of products on oxidation. Quinone is one of the oxidation products of phenol which form phenoquinone on reaction with phenol. Phenoquinone has brilliant red color.
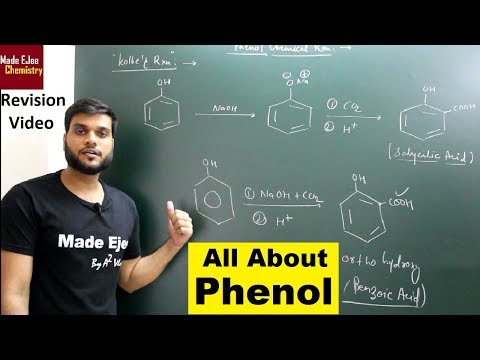
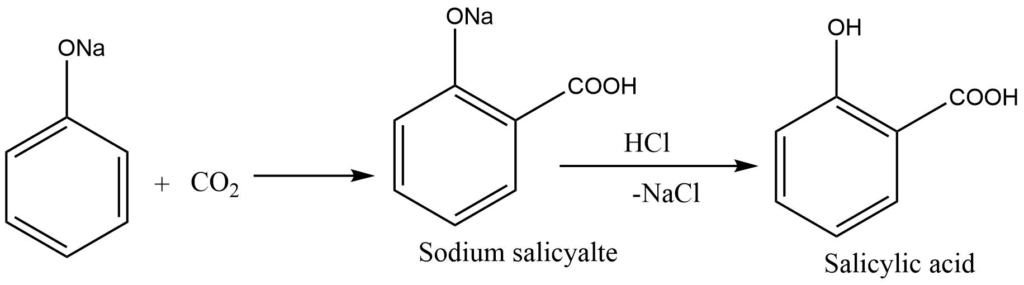
Kolbe’s reaction
Sodium phenoxide, on heating with CO2 at 135°C under 4 – 7 atm pressure, produces sodium salicylate, which on acidification with dilute HCl, gives salicylic acid. Salicylic acid acts starting material for the preparation of aspirin (an analgesic).
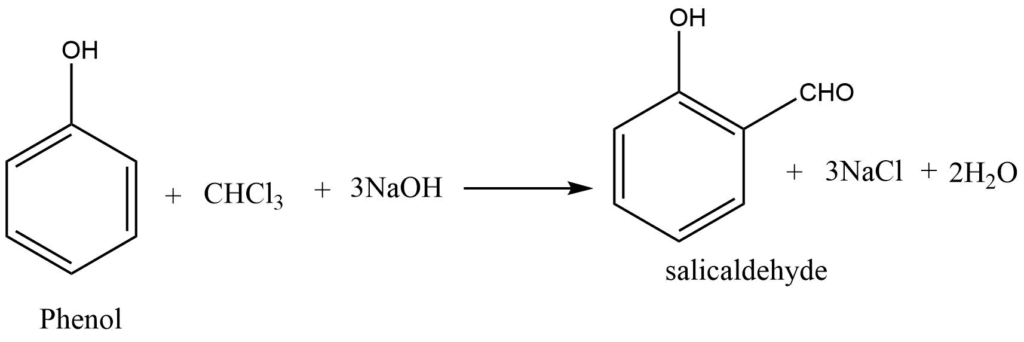
Reimer-Tiemann reaction
When phenol is heated in the presence of chloroform and alkali, salicylaldehyde is formed. This is known as the Reimer-Tiemann reaction.
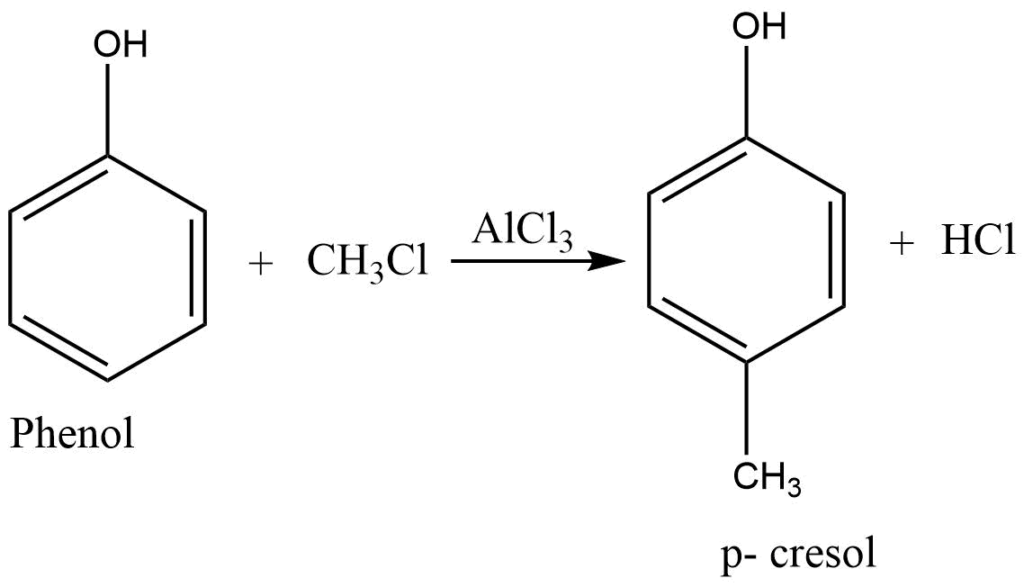
Friedel-Craft’s reaction
In the presence of anhydrous aluminium chloride, phenol reacts with an alkyl halide to form p-Cresol.
Reaction with FeCl3
With a drop of FeCl3, phenol turns violet due to the formation of colored complex iron salt.
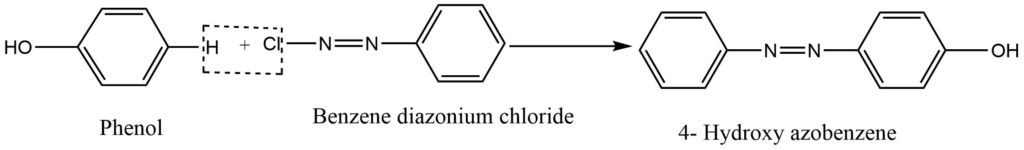
Coupling reaction
At low temperatures, benzene couples with benzene diazonium chloride in a slightly alkaline medium to form p-hydroxyazobenzene which is a red dye.
Condensation reactions
a. In the presence of concentrated sulfuric acid, phenol undergoes a condensation reaction with an acid anhydride to form phenolphthalein.
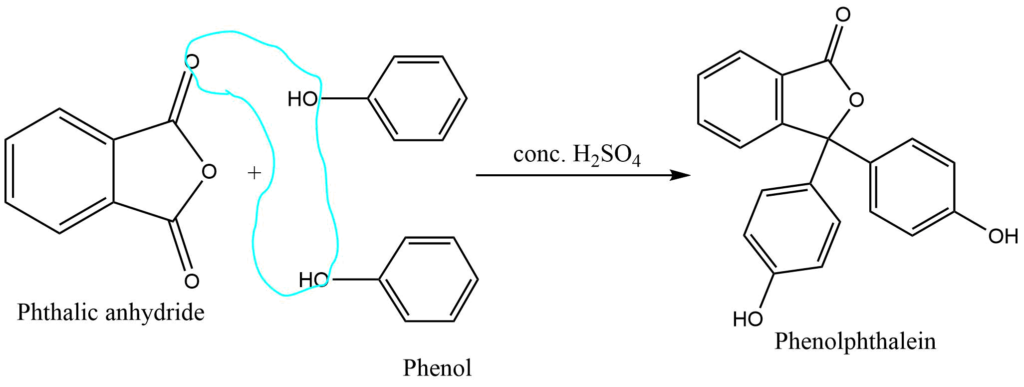
b. Condensation of phenol and formaldehyde in the presence of a basic catalyst form phenyl-formaldehyde resin. Methylene bridges are formed in ortho, para, or both positions during this reaction. Depending on the reaction conditions, both linear and cross-linked polymers are formed.
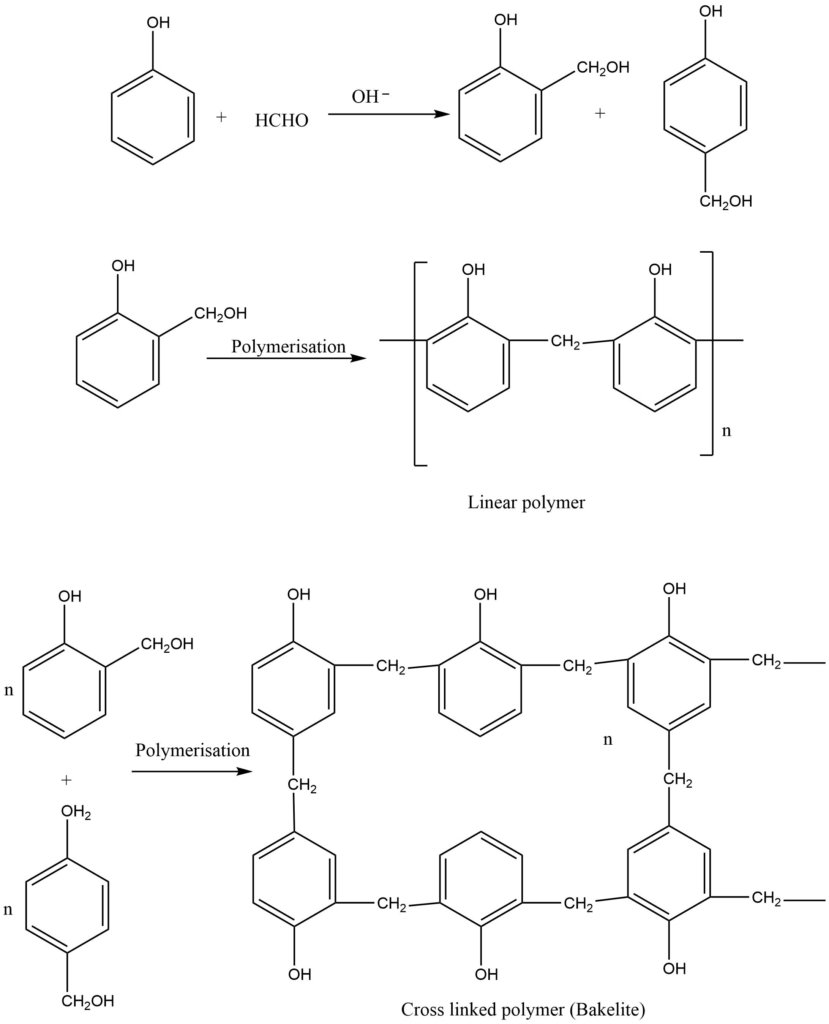
Bakelite makes phonograph records, combs, wire insulation, switches, and automobile distribution caps. In contrast, soft bakelite is used for binding glue for laminated wooden planks and in varnishes and lacquers. Sulphonate bakelites are used as ion exchange resin.
Reaction with ammonia
In the presence of the dehydrating agent, phenol reacts with ammonia to give aniline.
Uses of phenol
- It is used for the manufacture of Bakelite.
- It acts as row material for making salicylic acid and drugs like salol and aspirin.
- In low concentrations, phenol is used as a disinfectant in household cleaners and mouthwash.
- Cough drops and other antiseptics contain less toxic phenols like n-hexylresorcinol.
- Butylated hydroxytoluene is a common antioxidant in foods.
- Phenol is commonly used in ingrown toenail surgeries.
- It makes photographic developers, detergents, wood preservatives, and selective weed killers.
- Muscle spasticity can be treated with phenol injections.
Differences between aliphatic alcohol and phenol
| S.N. | Phenol | Alcohol |
| 1. | It has characteristic phenolic order. | Alcohols have a wine-like odour. |
| 2. | Acidic in nature. | They are neutral. |
| 3. | On reaction with FeCl3 it gives violet coloration. | They don’t react with FeCl3. |
| 4. | It forms dyes with the diazonium salt. | No action with a diazonium salt. |
| 5. | Phenol undergoes hydrogenation reaction. | Alcohols don’t undergo a hydrogenation reaction. |
| 6. | It does not dehydrogenate. | Alcohols undergo a dehydrogenation reaction to give aldehyde. |
Similarly between aliphatic alcohol and phenol
| S.N. | Phenol | Alcohol |
| 1. | Phenol on reaction with acetyl chloride or acid anhydride forms ester. | Alcohol also forms ester on reaction with acetyl chloride or acid anhydride. |
| 2. | The reaction of sodium phenoxide with alkyl halide produces ethers | The reaction of sodium alkoxide with alkyl halide also produces ethers |
| 3. | Phenols on reduction form aromatic hydrocarbon | Alcohols on reduction produce aliphatic hydrocarbon. |
Watch these videos for more information.
References
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- https://www.britannica.com/science/phenol
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Phenols
- https://byjus.com/chemistry/phenol/
- https://byjus.com/chemistry/phenols-nomenclcature/
- https://www.healthline.com/health/what-is-phenol#medical-uses
- https://www.vedantu.com/chemistry/phenol