The Aufbau principle is a crucial chemistry principle for determining an atom’s electron configuration in its ground state. The Aufbau principle requires the atom to adhere to Pauli’s exclusion principle and Hund’s rule, which states that each orbital can initially only hold one electron before becoming doubly occupied (no two electrons in one orbital have same spin).
Interesting Science Videos
What is the Aufbau Principle?
According to the Aufbau Principle, electrons will fill an atom’s lowest energy levels first and its highest energy levels last. There are four different types of subshell shapes and seven different energy levels that electrons can occupy. The Aufbau Principle contains historical predictions about the order in which they will fill.
The Aufbau principle thus specifies how electrons are positioned in an atom’s ground state atomic orbitals. Accordingly, electrons are inserted into atomic orbitals in ascending order of orbital energy. The Aufbau principle states that atomic orbitals that are available and have the lowest energy levels are filled before those that have higher energy levels.
History of Aufbau Principle
In the early 1920s, Niels Bohr and Wolfgang Pauli developed the Aufbau principle, which is a key concept in the new quantum theory.
This was an early attempt to explain chemical properties in terms of physics by applying quantum mechanics to the characteristics of electrons.
Before quantum mechanics, electrons were thought to be in classical elliptical orbits according to the old quantum theory. The “circular orbits” outside the inner electrons have the highest angular momentum, but the s- and p-subshell orbits have high subshell eccentricity, which brings them closer to the nucleus and causes them to experience, on average, a less strongly screened nuclear charge.
Salient Features of the Aufbau Principle
- The Aufbau principle states that electrons first occupy orbitals with the lowest energies. This suggests that electrons only move into orbitals with higher energies once orbitals with lower energies are fully occupied.
- The (n+l) rule, which states that the orbital’s energy level is determined by the sum of its principal and azimuthal quantum numbers, can be used to determine the order in which the energy of orbitals increases.
- Lower orbital energies are correlated with lower (n+l) values. The orbital with the lower n value is said to have lower energy associated with it if two orbitals have equal (n+l) values.
- The orbitals are filled with electrons in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Madelung’s Rule
The filling of atomic orbitals and electron configuration are described by Madelung’s rule.
Charles Janet first described Madelung’s rule or Klechkowski rule in 1929, and Erwin Madelung rediscovered it in 1936. V.M. Klechkowski described Madelung’s rule’s theoretical underpinnings. On Madelung’s rule, the modern Aufbau principle is hence based.
According to the rule:
(1) Energy increases as n + l increases.
(2) Energy rises with an increase in n for the same values of n and l.
Filling orbitals in the following order yields the following results:
1s, 2s, 2p, 3s, 3d, 4p, 5s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p (8s, 5g, 6f, 7d, 8p, and 9s)
The ground state of the heaviest atom known, Z = 118, does not contain any of the orbitals listed in parentheses. Because the inner electrons shield the nuclear charge, orbitals fill in this manner. Therefore, the orbital penetration: s > p > d >f
Steps for Aufbau Diagram
- Firstly, find out how many electrons are present in the atom.
- Add the first two electrons to the s orbital in the first energy level (1s orbital).
- Insert the second two electrons into the s orbital of the second energy level (2s orbital).
- Start by placing one electron in each of the three p orbitals in the second energy level (2p orbitals), and if there are still electrons available, go back and then add a second electron to complete the electron pairs.
- Keep going in this manner through each subsequent energy level until all of the electrons have been distributed.
Electron Filling in Subshells
- An electron’s state is indicated by a quantum number. Four quantum numbers are present, two of which are related to the subshells. They are the azimuthal quantum number l and the principal quantum number n.
- The order of the subshells’ increasing energies is determined by the sum of n and l. According to the rising value of (n+l), the electrons fill up the subshell. The values of n and l are discrete, such as n = 0, 1, 2,… and l = 0, 1, 2, and 3. For instance, the 3p subshell is referenced by n = 3 and l = 1.
- The electron will reside in the lower n subshell if two subshells have the same (n+l) value. For instance, the (n+l) value of 3p and 4s is equal to 4. The electron will be occupied by 3p rather than 4s because 3p has a lower n value.
The order of filling the subshells
| Subshell | Principle quantum number (n) | Azimuthal quantum number (l) | Sum (n + l) |
|---|---|---|---|
| 1s | 1 | 0 | 1 |
| 2s | 2 | 0 | 2 |
| 2p | 2 | 1 | 3 |
| 3s | 3 | 0 | 3 |
| 3p | 3 | 1 | 4 |
| 4s | 4 | 0 | 4 |
| 3d | 3 | 2 | 5 |
| 4p | 4 | 1 | 5 |
| 5s | 5 | 0 | 5 |
| 4d | 4 | 2 | 6 |
| 5p | 5 | 1 | 6 |
| 6s | 6 | 0 | 6 |
| 4f | 4 | 3 | 7 |
| 5d | 5 | 2 | 7 |
| 7s | 7 | 0 | 7 |
| 5f | 5 | 3 | 8 |
| 6d | 6 | 2 | 8 |
| 7p | 7 | 1 | 8 |
| 8 | 8 | 0 | 8 |
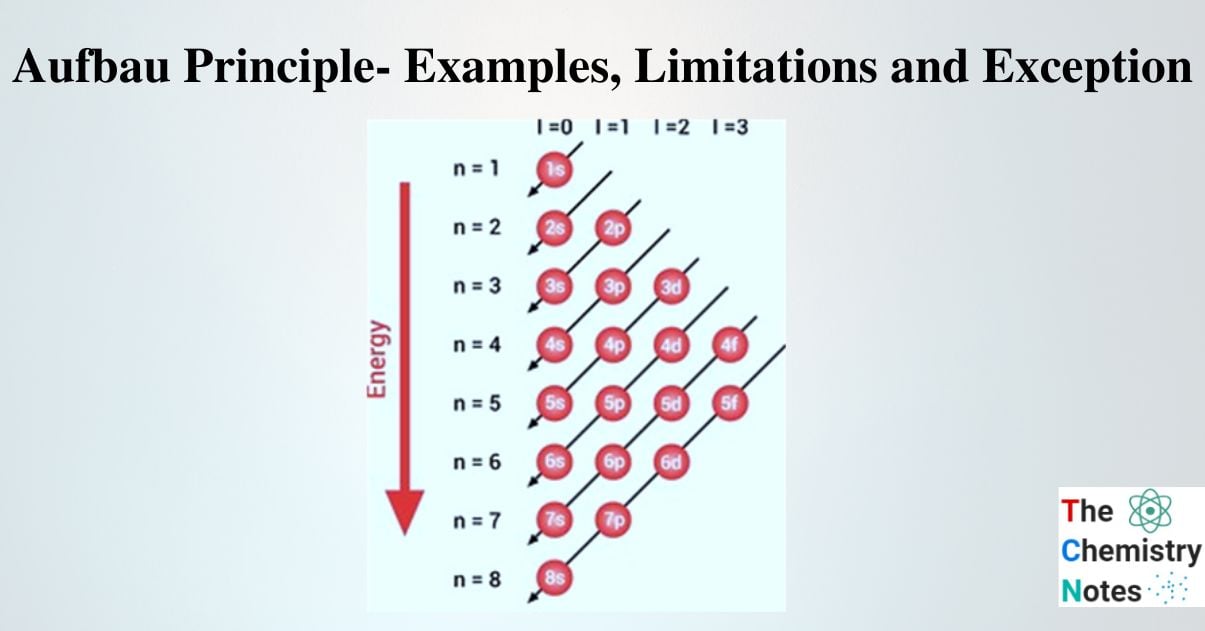
Construct Aufbau Diagram
The Aufbau principle states that as (n + l) values increase, the order of filling the subshells gets higher. Therefore, a diagram known as the Aufbau diagram can serve as a representation of this information. Hence, to build the diagram, the points listed below are taken into consideration.
- A column on the left lists the values of n whereas, on the top, l values are listed in a row.
- Subshells are thus represented by the sum of the n and l values.
- Diagonal lines are drawn to connect subshells with the same (n + l) value.
- There are arrows that point in the direction of rising n on the diagonals.
![Aufbau Diagram [Aufbau Principle]](https://scienceinfo.com/wp-content/uploads/2022/11/image-166.png)
Electronic Configuration Using the Aufbau Principle
The 1s orbital, which is the smallest and has the lowest energy, eventually comes first in the filling order. As a result, the first electron enters the 1s orbital, giving the hydrogen atom its 1s1 electronic configuration. The second electron then follows, and since the s orbital can accommodate two electrons, it also enters the 1s orbital. The electronic configuration is now 1s2 which is thus the inert gas helium. The atomic number rises by one for every electron that is added because a proton is also added.
The third electron enters the second orbital after the first one is filled, giving the lithium atom, which is located directly beneath hydrogen in the periodic table, its electronic configuration of 1s2 2s1. Therefore, the same is true for beryllium, 1s2 2s2.
Electronic Configuration based on the Aufbau principle
| Element | Symbol | Atomic number (Z) | Electronic configuration |
|---|---|---|---|
| Hydrogen | H | 1 | 1s1 |
| Helium | He | 2 | 1s2 |
| Lithium | Li | 3 | 1s2 2s1 |
| Carbon | C | 6 | 1s2 2s2 2p2 |
| Oxygen | O | 8 | 1s2 2s2 2p4 |
| Neon | Ne | 10 | 1s2 2s2 2p6 |
| Sodium | Na | 11 | 1s2 2s2 2p6 3s1 |
| Magnesium | Mg | 12 | 1s2 2s2 2p6 3s2 |
| Phosphorus | P | 15 | 1s2 2s2 2p6 3s2 3p3 |
| Argon | Ar | 18 | 1s2 2s2 2p6 3s2 3p6 |
| Potassium | K | 19 | 1s2 2s2 2p6 3s2 3p6 4s1 |
| Scandium | Sc | 21 | 1s2 2s2 2p6 3s2 3p6 4s2 3d1 |
| Iron | Fe | 26 | 1s2 2s2 2p6 3s2 3p6 4s2 3d6 |
| Bromine | Br | 35 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 |
| Krypton | Kr | 36 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 |
| Zirconium | Zr | 40 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d2 |
| Tin | Sn | 50 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2 |
| Plutonium | Pu | 94 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f6 |
Aufbau Principle Exceptions
The Aufbau principle does not hold for all atoms in general. Spectroscopic research has revealed that some atoms defy the Aufbau principle.
Transition Metals, Lanthanides, and Actinides
Transition metals, lanthanides, and actinides, in particular, have electron configurations that contradict the Aufbau principle. The findings include the following observations.
- The s subshell loses an electron to the d subshell in transition metals. However, Palladium is an exception, where the 5s subshell loses two electrons to the 4d subshell. The picture below serves as an example of this exception.
- In some lanthanides and actinides, an electron from the f subshell is consumed by the d subshell. Except for thorium, as the 6d subshell in thorium absorbs two electrons from the 5f shell.
This is due to a variety of factors, including the increased stability of half-filled subshells and the relatively low energy difference between the 3d and 4s subshells.
![Aufbau Exception of Palladium [Aufbau Principle]](https://scienceinfo.com/wp-content/uploads/2022/11/image-167.png)
Exception of Aufbau Principle in Transition Elements
| Element | Symbol | Atomic number | Aufbau’s prediction | Experimental observed |
|---|---|---|---|---|
| Chromium | Cr | 24 | [Ar] 4s2 3d4 | [Ar] 4s1 3d5 |
| Copper | Cu | 29 | [Ar] 4s2 3d9 | [Ar] 4s1 3d10 |
| Niobium | Nb | 41 | [Kr] 5s2 4d3 | [Kr] 5s1 4d4 |
| Molybdenum | Mo | 42 | [Kr] 5s2 4d4 | [Kr] 5s1 4d5 |
| Ruthenium | Ru | 44 | [Kr] 5s2 4d6 | [Kr] 5s1 4d7 |
| Rhodium | Rh | 45 | [Kr] 5s2 4d7 | [Kr] 5s1 4d8 |
| Palladium | Pd | 46 | [Kr] 5s2 4d8 | [Kr] 4d10 |
| Silver | Ag | 47 | [Kr] 5s2 4d9 | [Kr] 5s1 4d10 |
| Platinum | Pt | 78 | [Xe] 6s2 4f14 5d8 | [Xe] 6s1 4f14 5d9 |
| Gold | Au | 79 | [Xe] 6s2 4f14 5d9 | [Xe] 6s1 4f14 5d10 |
Exception of Aufbau Principle in Lanthanide and Actinide
| Element | Symbol | Atomic number | Aufbau’s prediction | Experimentally observed |
|---|---|---|---|---|
| Lanthanum | La | 57 | [Xe] 6s2 4f1 | [Xe] 6s2 5d1 |
| Cerium | Ce | 58 | [Xe] 6s2 4f2 | [Xe] 6s2 4f1 5d1 |
| Gadolinium | Gd | 64 | [Xe] 6s2 4f8 | [Xe] 6s2 4f7 5d1 |
| Actinium | Ac | 89 | [Rn] 7s2 5f1 | [Rn] 7s2 6d1 |
| Thorium | Th | 90 | [Rn] 7s2 5f2 | [Rn] 7s2 6d2 |
| Protactinium | Pa | 91 | [Rn] 7s2 5f3 | [Rn] 7s2 5f2 6d1 |
| Uranium | U | 92 | [Rn] 7s2 5f4 | [Rn] 7s2 5f3 6d1 |
| Neptunium | Np | 93 | [Rn] 7s2 5f5 | [Rn] 7s2 5f4 6d1 |
| Curium | Cm | 96 | [Rn] 7s2 5f8 | [Rn] 7s2 5f7 6d1 |
| Lawrencium | Lr | 103 | [Rn] 7s2 5f14 6d1 | [Rn] 7s2 5f14 7p1 |
Heavy Nuclei
The Aufbau principle is also violated by heavy nuclei (Z > 120). High electrostatic forces pull the electrons toward the nucleus as its charge rises. Their speeds are almost equal to the speed of light when it occurs. Hence, determining the electron energies using a quantum mechanical approach is unsuccessful.
Ruthenium
Ruthenium is primarily used as an alloying component to harden platinum and palladium. The distribution of electrons in Ru is as follows: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d7 5s1.
The energy of the 5s orbital in this meta is lower than that of the 4d orbital (n+l for 4d is 7 and for 5s is 5). However, the 4D orbit will begin to fill up before the 5S orbit. Therefore, rather than [Kr] 4d6 5s2, Ru’s electronic configuration is [Kr] 4d7 5s1.
Rhodium
Rhodium (Rh) also violates the Aufbau principle, just like Ruthenium does. It has the atomic number 45. Despite having less energy than 5s, the 4d orbital in Rh fills up before the 5s orbital does. Rhodium has the following electronic configuration: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d8 5s1. The configuration is [Kr] 4d7 5s2 if the Aufbau principle is followed.
Limitations of Aufbau Principle
According to Aufbau’s principle, electrons first occupy atomic orbitals in the lowest possible energy state before moving up to the higher level. But this has some restrictions, just like other ideas.
- Similar to the d and f block elements, which add stability to the atoms whether they are filled or partially filled, do not always follow the Aufbau Principle.
- The Aufbau Principle states that the order of orbital energies between different and given elements is always fixed, but this is not entirely accurate. It is accurate up to a point and somewhat helpful. Only two electrons can be positioned into atomic orbitals with fixed energy, according to the Aufbau Principle. The energy of the electrons in an atomic orbital depends on the total energy of the atom’s electrons.
- Secondly, as we know, a hydrogen-like atom has only one electron, and the energy in the s-orbital and p-orbital shells is the same. However, the nucleus of an actual hydrogen atom has a different number of protons. Thus the magnetic field of the nucleus slightly splits the energy levels, changing the energy of each electron in the process.
- The Aufbau Principle is extremely effective for the ground state of the atoms up to the first 18 elements, but it is less effective for the remaining 100 electrons after that.
Examples
- The first two electrons of lithium occupy the 1s orbital, and the third electron moves into the 2s orbital, the next lowest level that is available. The electron configuration of lithium is 1s2 2s1.
![Lithium [Aufbau Principle]](https://scienceinfo.com/wp-content/uploads/2022/11/image-168.png)
- The 2p sublevel’s occupancy is finished with the tenth electron. The electron arrangement of neon is 1s2 2s2 2p6. The second energy shell of neon, whose primary quantum number is 2, is full. Due to its unusually stable configuration, neon is thus not chemically reactive.
![Neon [Aufbau Principle]](https://scienceinfo.com/wp-content/uploads/2022/11/image-169.png)
References
- https://www.chemistrylearner.com/aufbau-principle.html
- https://www.coursehero.com/study-guides/introchem/the-building-up-aufbau-principle/
- https://chemistrygod.com/aufbau-principle
- https://protonstalk.com/atom/aufbau-principle/
- https://byjus.com/chemistry/aufbau-principle/
- https://collegedunia.com/exams/aufbau-principle-electronic-configuration-exceptions-and-limitations-chemistry-articleid-585
- https://www.adda247.com/school/aufbau-principle/
- Feynman, Richard; Leighton, Robert B.; Sands, Matthew (1964). “19. The Hydrogen Atom and The Periodic Table”. The Feynman Lectures on Physics. Vol. 3. Addison–Wesley
- Miessler, Gary L.; Tarr, Donald A. (1998). Inorganic Chemistry (2nd ed.). Prentice Hall.

It was really helpful. Looking for other articles like it. Thank you