
Azo compounds are the compounds of both natural and manufactured chemicals that contain at least one azo group (N=N) in their structure. The atomic groups connected to the nitrogen atoms might be of any organic class, but the commercially relevant azo compounds, which account for more than half of all commercial dyes, contain the benzene group or its derivatives as the attached groups.
Although azo compounds come in a wide variety, pigments, and dyes are the most popular. Bismarck Brown, the first commercial azo dye, was introduced in 1863. This resulted in a considerable increase in the use of azo compounds throughout the 1880s, providing a diverse range of additional azo colors principally employed in the textile sector.
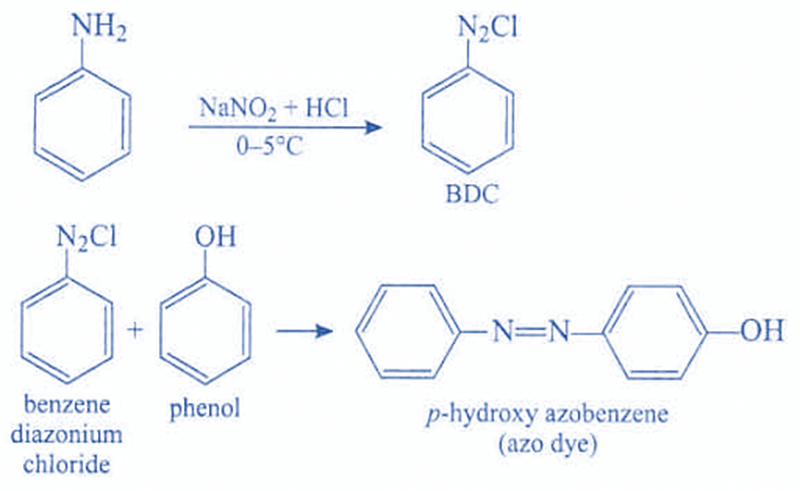
The majority of aromatic azo compounds are produced by reacting a diazonium salt with an organic molecule containing easily replaceable hydrogen atoms. Another useful method for symmetrical azo compounds is the separation of azobenzene from nitrobenzene using particular oxygen-removing chemicals.
The aliphatic organic groups, produced by dehydrogenating the corresponding hydrazo compounds (having the group —HN—NH—) produced from hydrazine, N2H4, are used to form the attached groups in azo compounds.
The chemical structure of azo dyes consists of a dominating auxochrome group, a chromophoric group, and solubilizing groups. The azo bond and the related chromophores and auxochromes determine the color of the dye. Azo dyes are common in the textile, printing, and other industries. They account for 70% of all commercial dyes on the market.
Interesting Science Videos
Preparation of azo dyes
Azo dyes are made using a simple diazotization and coupling procedure. Their significance has grown dramatically. Azo dyes now account for more than half of all commercial dyes and have an advantage in the printing press market.
Azo dyes are distinguished by chemical groups that can establish covalent connections with textile substrates. The diazotization of a primary amine aromatic and coupling with a diazonium salt is the most typical way of synthesis.
Two organic compounds—a coupling component and a diazo component are necessary for making an azo dye. An enormous range of potential dyes is available because these can be significantly altered, especially because the basic molecules are readily available and affordable.
Furthermore, because the reactions are simple, the method may be easily scaled up or down, which is usually an important consideration in the cost of chemicals. Since most of the chemical reaction occurs at or below room temperature, the reaction requires little energy. Because all reactions occur in water, which is simple and inexpensive to collect, purify, and dispose of, the effect on the environment is also minimized. Azo dyes are increasingly appealing alternatives when other dye classes lose their viability for environmental or economic reasons. Some azo dye preparation methods include:
- Azo dyes are typically made by diazotizing a primary aromatic amine and then coupling it with one or more electron-rich nucleophiles. Amino and hydroxy are two examples of electron-rich nucleophiles.
2. Another way to make azo dyes is to reduce nitroaromatic derivatives in an alkaline media.
3. It can also be made by oxidizing primary amines with potassium permanganate.
4. Nitroso compound reduction using lithium aluminum hydride. also produce azo dyes
5. Nitroso derivatives and primary amine condensation produce azo dyes.
Azo dyes are characterized by chemical groups having the ability to form covalent bonds with textile substrates. The most common method of production is the diazotization of a primary amine aromatic and the coupling with a diazonium salt.
The general formula for making an azo dye requires two organic compounds- a coupling component and a diazo component. Since these can be altered considerably, an enormous range of possible dyes is available, especially as the starting molecules are readily available and cheap.
Furthermore, the simplicity of the reactions means that the process can be scaled up or down very easily, which is always a key factor in the cost of chemicals. Energy requirements for the reaction are low since most of the chemistry occurs at or below room temperature. The environmental impact is reduced by the fact that all reactions are carried out in the water, which is easy and cheap to obtain, clean, and dispose of. As other dye classes become less viable for either environmental or economic reasons, azo dyes become ever more attractive options.
Types of Azo compounds
Azo dyes are the most significant category of synthetic colorants and the most prevalent class of dyes in use today. There are different systems of classification for azo dyes. Disperse dyes, such as Disperse Orange, are included in some classes. Another classification method for azo dyes is based on the number of azo groups. The majority of azo dyes have only one azo group (monoazo), but some have two (diazo), four (tetrakis azo), or more.
Types of azo dyes based on the presence of an aromatic or aliphatic group
Aromatic azo dyes
Aryl azo compounds are typically crystalline and stable. Azobenzene is the most common aromatic azo compound. It exists mostly as the trans isomer but changes to the isomer upon photolysis. Aromatic azo compounds can be produced through azo coupling, which involves an electrophilic substitution reaction in which an aryl diazonium cation is attacked by another aryl ring, particularly those substituted with electron-donating groups.
ArN2+ + Ar’H → ArN = NAr’ + H+
Since diazonium salts are typically unstable at ambient temperature, azo coupling reactions are typically carried out near 0 °C. Additionally, azo compounds are produced through the oxidation of hydrazines (RNHNHR′). Azo dyes are also generated by combining nitroaromatics with anilines and then reducing the resultant azoxy intermediate
Chemical reaction:
ArNO2 + Ar’NH2 → ArN(O) = NAr′ + H2O
ArN(O) = NAr′+C6H12O6 → ArN = NAr′ + C6H12O6 + H2O
Aromatic azo compounds (R = R’ = aromatic) are often stable and have vibrant colors like red, orange, and yellow. As a result, they are employed as dyes known as azo dyes. Because of their capacity to serve as weak acids and the distinct colors of the acid and salt forms, some azo compounds, such as Methyl orange, can also be employed as acid-base indicators. Another common aromatic azo compound is azobenzene. Their color comes from absorbance in the visible area of the spectrum caused by electron delocalization in the benzene and azo groups, which form a conjugated system with the N=N group as part of the chromophore.
Aliphatic azo compounds
Azo compounds with aliphatic organic groups as attached groups are often formed by dehydrogenation of the equivalent hydrazo compounds (having the group HNNH) derived from hydrazine, N2H4. The breakdown of aliphatic azo compounds by heat into nitrogen and free radicals is an important reaction; the latter are frequently employed to start polymerization reactions.
For instance, EtN=NEt, diethyl diazene.
The carbon-nitrogen (C-N) bonds are broken when exposed to high temperatures or irradiation. Certain alkyl azo compounds cleave with the loss of nitrogen gas to produce radicals, which are mostly utilized as an initiator in free-radical polymerizations and other radical-induced processes. It began by breaking down, removing a molecule of nitrogen gas, then producing two 2-cyanoprop-2-yl radicals:
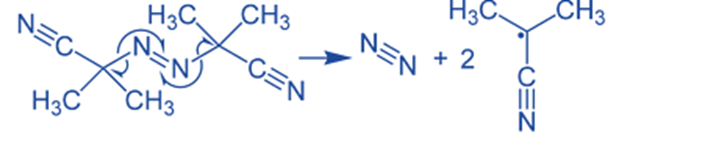
2-cyanoprop-2-yl radicals
Azobisisobutylonitrile (AIBN) is synthesized by converting acetone cyanohydrin to the hydrazine derivative and then oxidizing it.
2(CH3)2C(CN)OH + N2H4 → [(CH3)2C(CN)]2N2H2 + H2O2
[(CH3)2C(CN)]2N2H2 + Cl2 → [(CH3)2C(CN)]2N2 + HCl
Types of azo dyes based on the number of azo groups present in the compound
Monoazo dyes
An old analog of this family that dyes cotton is a monoazo molecule with the general structure R -N=N- R’, R and R’ are the benzene or heterocyclic derivatives, which are characterized by their orange color. Orange dye, for example, is used to color cellulose acetate, polyamides, polyesters, and polyacrylonitrile.
Diazo dyes
In general, diazo dyes are composed of two groups -N=N-. The blue direct dye, for example, has a benzidine function in its molecule.
Polyazo dyes
Polyazo dyes are complex dyes containing the azo group repeated three or more times in the same molecule. They are designed for dyeing leather in dark colors such as red, brown, and dark black. Direct red is the most commonly used dye for this type of dye.
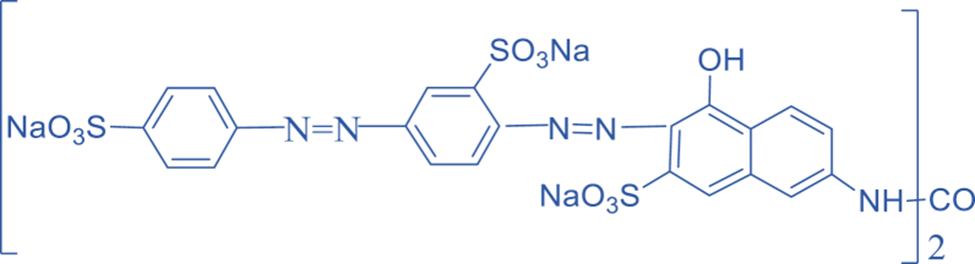
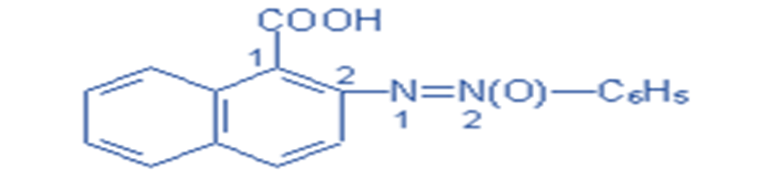
Azoxy compound
The parent hydride, RH, is substituted by the -azoxy group in an azoxy compound with a general structure of RN = N(O)R’ or RN(O) = NR’, in which R is substituted by a principal characteristic group. The position of the oxygen atom is indicated by the prefix NNO-, ONN-, or NON-.
Reactive dyes
These dyes are referred regarded as “reactive” because they chemically react with the fiber molecules to establish a covalent bond, which permanently bonds the dye molecules to the fiber.
Reactive dyes are favored over other types of dyes because they provide a wide spectrum of bright and vivid colors, excellent color fastness, and outstanding wash and light fastness. Reactive dyes can also be used to produce a wide range of colors, ranging from pastel to intense.
A chromosphere in a reactive dye comprises a substituent that reacts with the substrate. Due to the bonding that occurs after dyeing, reactive dyes offer strong fastness qualities. Reactive dyes are most typically employed in the dyeing of cellulose, such as cotton or flax, but wool can also be dyed with reactive dyes.
By Depending on Chemical Constitution:
Depending on chemical constitution reactive dyes can be classified as:
- Chlorotriazine Dyes (MCT)
- Vinyl Sulphone Dyes (VS)
- Heterocyclic Halogen Containing Dyes (HHC)
- Mixed Dyes (MCT-VS)
Chlorotriazine Dyes
Monofunctional Chlorotriazine Dyes
Monochlorotriazine
At temperatures ranging from 20°C to 30°C, these colors exhibit a high affinity for cellulose. The replacement of a single chlorine by the hydroxyl ion or the cellulose ion results in a significant decrease in the reactivity of the second chlorine. In an alkaline media, ionization of the hydroxyl group causes the negative charge of the triazine ring atoms to be relocated, while the chlorine atom is inactivated and the carbon that links to the chlorine becomes less electrophilic. They have good substantive properties for fibers and a high degree of fixation efficiency.
Bifunctional Chlorotriazine Dyes
These dyes have excellent fastness qualities and contain two reactive groups. Low temperatures are required for dye uptake, resulting in pale hues. Two reactive groups are carried by bifunctional dyes. (Bifunctional dyes, both homo, and hetero). They are well-known for their high dyeing efficiency and overall fastness.
They provide improved repeatability, particularly for medium to pale hues.
Dichlorotriazine is a very easy dye to work with; there is no need to steam or otherwise heat-set the fabric.
These dyes are relatively safe. They have amazing wash fastness.
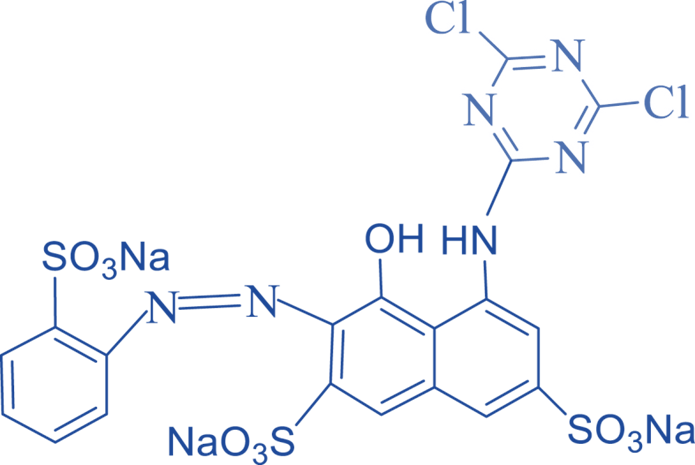
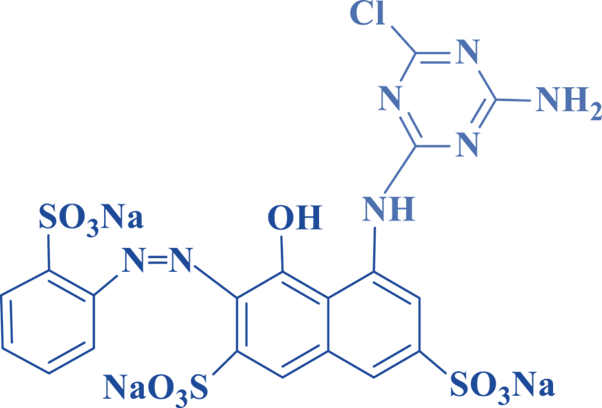
Mono-amino-chlorotriazine
It shares the chromophoric groups with dichloro triazine. It contains active chlorine and the NH2 group. The -NH- connection between the chromophore and the reactive group influences the dyeing characteristics and solubility.
Vinyl Sulphone Dyes (VS)
The reactive component of these cores contains the -sulfatoethylsulphone functional group (SO2- (CH2)2 -O-SO3Na). These dyes had a modest affinity as compared to halohydrocyclics. It has a high water fastness in acidic media. Because of the presence of the group (-O-SO3Na), the sulphatoethylsulfone group results in better water solubility. After the sulfone group is removed in an alkaline medium, the solubility decreases, and the affinity for cellulose increases.
Heterocyclic Halogen Containing Dyes (HHC)
Pyrimidines In general, include the following di and tri-chor pyrimidine derivatives, chlorfluoropyrimimidine, and fluoropyrimidine derivatives:
Chlorofluoropyrimidine
The substitution of fluorine for chlorine increased the reactivity of this type of dye. In an acid media, the bond formed with the textile fiber is more stable; nevertheless, in the presence of light, this bond becomes susceptible to oxidation by the peroxide component.
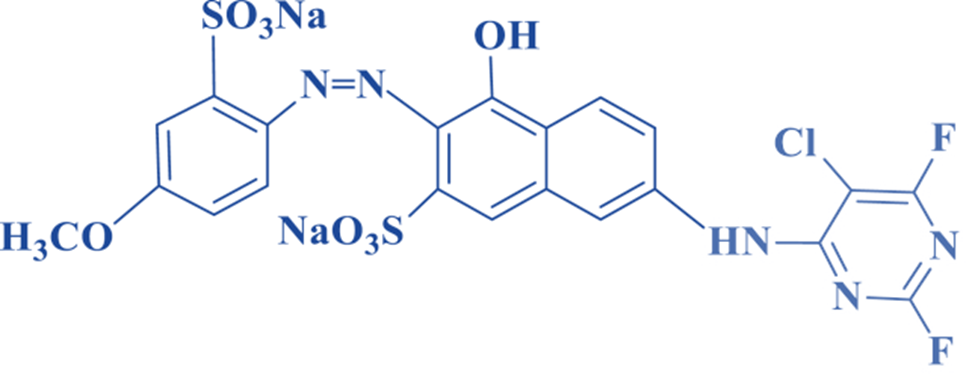
Dichloroquinoxaline
This dye has high reactivity to dichloro pyrimidine dyes, dichloro triazine dyes, and difluoro pyrimidine dyes. The fiber-dye bond has been found to be less resistant to strength when exposed to peroxides, light, and/or heat.
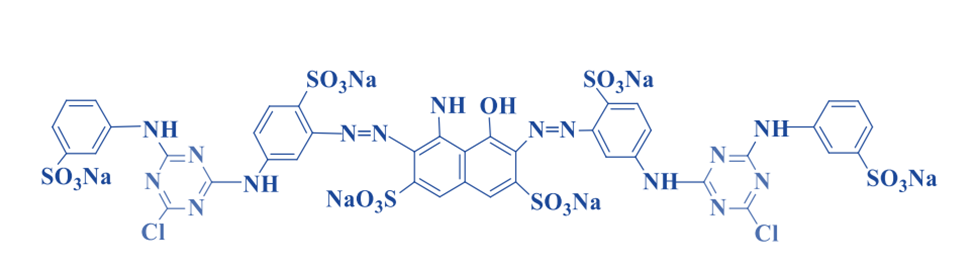
Mixed Dyes (MCT-VS)
Monochlorotriazine –sulphatoethylsulphone
The interaction of dichloro triazine with an arylamine bearing the sulphatoethylsulfone group can produce monochlorotriazine-sulphatoethylsulfone. The reactive groups promote cellulosic fiber adhesion. Because of their high affinity, triazine induces sulphatoethylsulfone to adsorb fibers in a bifunctional state. The presence of two types of fiber-dye linkages encourages varying levels of rigidity.
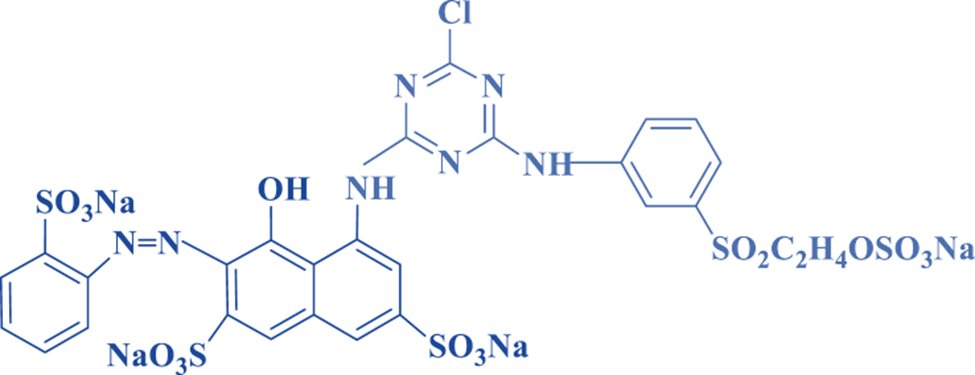
Properties of Azo dyes
Azo dyes produce substantially brighter, more intense colors than the next most prevalent dye class (anthraquinones).
They have fair to good fastness properties.
Color fastness
It is the tendency of a pigment or dye, or the leather, cloth, paper, ink, etc., containing the coloring matter, to maintain its original color, particularly without fading, running, or changing when wet, washed, cleaned; or stored under normal circumstances when exposed to light, heat, or other influences.
Geometrical isomerism
Azo dyes show geometrical isomerism. The planar -N=N- bond, like any other double bond, exhibits geometrical isomerism:
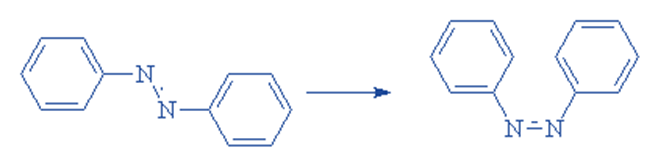
Exposure to UV light can cause this transition from trans (preferred) to cis. This can result in photochromism, a reversible color change caused by light in some dyes.
Tautomerism
This entails removing hydrogen from one region of the molecule and adding hydrogen to another part of the molecule. This is frequent when the azo group has a -OH group ortho or para to it. Tautomeric forms can be distinguished by their characteristic spectra. In comparison to their hydroxy azo counterparts, keto hydrazones are typically bathochromic.
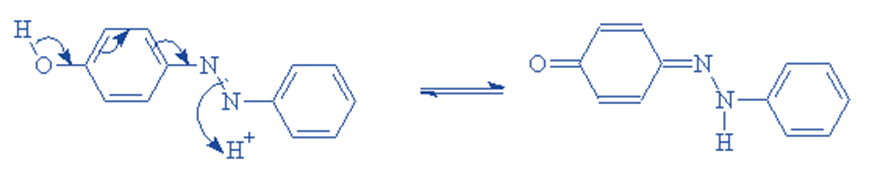
Reactive dyes offer a high degree of fabric attachment, resulting in bright and vibrant colors.
Even after repeated washing or exposure to sunshine, reactive dyes are highly resistant to fading or bleeding.
Applications of azo dyes
i. Azo dyes are important because of their distinct complexing capabilities, sensitivity as chromogenic reagents, and application in the spectrophotometric detection of various metal ions.
ii. Azo dyes are commonly utilized to color pharmacological substances in the pharmaceutical sector.
iii. Azo groups with cyclized-cyanine derivatives (ACC1 and ACC2) are employed in two-photon photodynamic therapy (cancer treatment).
iv. They are employed as a complexometric indicator and change color in the presence of metal ions (such as calcium and magnesium).
v. In addition, azo dyes are utilized in tattoos, printer inks, insecticides, pesticides, paints, varnishes, lacquers, and personal care products.
vi. They are commonly used for dyeing purposes in the textile sector.
vii. They are utilized in cosmetics, paper, plastics, and food, among other things.
viii. Azo dyes, particularly heterocyclic azo dyes, are an important type of organic chemical used in the detection of metal ions due to their high photometric sensitivity.
ix. They are used in hypnotic medication (to cure insomnia).
x. Considering the way azo compounds respond to light, there are numerous novel applications of them in agrochemicals. For example, azobisisobutyronitrile (AIBN), an alkyl rather than an aromatic azo molecule, is used as a radical initiator in a wide range of processes.
xi Azo dyes can also be employed to increase drug delivery or for cellular staining to aid in cell research.
Examples of some azo dyes
Methyl orange
Methyl Orange, usually designated as MeO, is an azo dye that is commonly used as a pH indicator in titrations of acid and base neutralization reactions. It has a pKa value of 3.47.
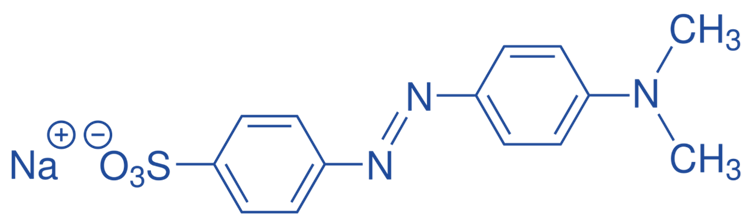
This is due to the fact that it exhibits apparent and distinct color variations at different pH levels.
- It has red color at pH < 3.1
- It has yellow color at pH > 4.4
- It shows orange color at pH between 3.1 and 4.4
MeO is carcinogenic because it has mutagenic qualities, which means it can induce temporary or permanent alterations in our DNA, which can lead to cancers.
Erichrome Black T
A complexometric titration’s end point can be precisely determined using the metal ion indicator ie., Erichrome black T. It is also known as solo chrome black and is frequently used as an indicator in complexometric titration.
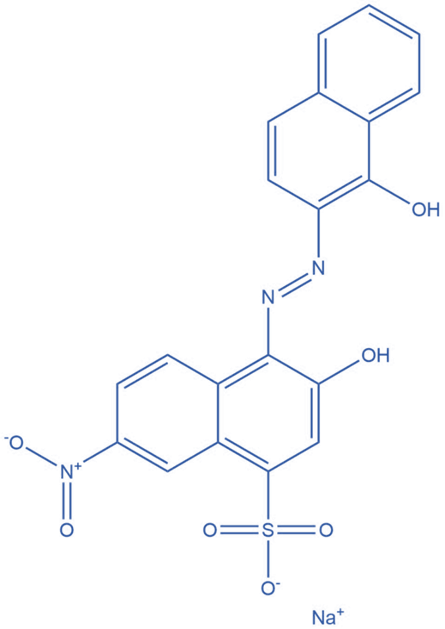
It is sodium 1-hydroxyl-2-naphthylazo-6-nitro-2-naphthol-4-sulfate. It has two sensitive color changing areas and exhibits acid-base behavior. Erichrome black T is used in complexometric titration to do metal ion titration using EDTA.
The indicator initially produces a colorful complex with metal ions that are stronger than it. The EDTA then replaces the indicator and creates a metal-EDTA complex, leaving the indicator colorless. A color change is detected when all of the indicators are moved by EDTA that indicate the end point.
M + In → M -In Complex
M -In Complex + EDTA → M-EDTA complex + In
Congo red
Congo red is an azo dye that is made from benzidine. Congo red was once used to color cotton but it has been replaced with dyes that are more resistant to light and washing. Congo red has two azo groups and is used as a pH indicator in titrations. It changes color as the pH varies.
It has blue color at pH < 3.0
It shows red color at pH < 5.0
The hazardous effect of azo dyes
i. Azo dyes show several harmful effects they are:
ii. Some azo dyes are carcinogenic in nature.
iii. They are reported as reproductive toxins.
iv. They have cellular and neurological toxicants.
v. They show allergic effect and acts as irritants,
vi. The color of dyes interferes with the photosynthesis process.
vii. They are poisonous to aquatic life.
References
- https://www.jchemrev.com/article_154655_047ad6f54ac616875de45d65020d94ec.pdf.
- https://www.sciencedirect.com/science/article/abs/pii/B9780128156476000042.
- https://www.britannica.com/science/azo-compound,
- https://www.vedantu.com/chemistry/azo-compound.
- https://www.chm.bris.ac.uk/webprojects2002/price/azo.htm.
- https://www.meghmaniglobal.com/what-are-reactive-dyes-types-of-reactive-dyes/.
- https://www.researchgate.net/publication/251464037_Heterocyclic_Dyes_Preparation_Properties_and_Applications#:~:text=Pyrazine%20is%20one%20of%20the,wide%20range%20of%20commercial%20applications.
- https://www.acdlabs.com/iupac/nomenclature/93/r93_377.htm.
- https://textilefashionstudy.com/reactive-dyes-definition-classification-properties-and-influencing-factors/
