Baking soda, chemically known as sodium bicarbonate, is a white crystalline powder with alkaline properties. It is a chemical compound with molecular formula, NaHCO3. Baking soda is a salt composed of a sodium cation (Na+) and bicarbonate anion (HCO3–).

It is commonly used in baking and household cleaning. It is used in baking as a leavening agent. It reacts with acidic ingredients to produce carbon dioxide gas, which helps dough rise and produce soft baked goods. Additionally, it has various household uses, such as cleaning.

Interesting Science Videos
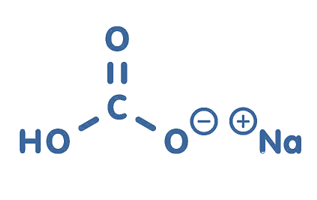
Structure of Baking Soda
Baking soda, chemically known as sodium bicarbonate (NaHCO3) has a simple chemical structure, which consists of sodium ions (Na+) and bicarbonate ions (HCO3–). These ions contribute to its versatility, allowing it to act as a leavening agent in baking.
Preparation of Baking Soda
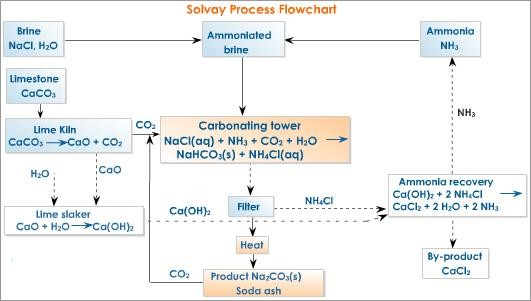
Baking soda or sodium bicarbonate is generally produced through a chemical process called the Solvay method. In this process, carbon dioxide, water, and ammonia, and brine solution in its concentrated form, are used a raw materials. The methods of preparation of baking soda through Solvay process are as follows:
- Sodium chloride (table salt) reacts with ammonia and carbon dioxide in the presence of water. The reaction occurs as:
NaCl + CO2 + H2O+ NH3 → NaHCO3 + NH4Cl………………..Eqn. i
2 NaHCO3 → Na2CO3 + CO2 + H2O……………………………….Eqn. ii
- Sodium bicarbonate (NaHCO3) is formed in the reaction, which is then precipitated and separated.
- Thus separated sodium bicarbonate is purified.
- The resultant carbon dioxide, which is produced in eqn. ii, is recycled to produce NaHCO3.
In this way, the white crystalline powder known as baking soda is formed by Solvay method.
Benefits of preparation of Baking soda by Solvay Method
The following are the benefits of preparation of baking soda by Solvay method:
- Cost-Effective: It is a cost-effective method because it utilizes the readily available raw materials such as sodium chloride (table salt) and ammonia.
- Efficiency: It is the most efficient and established industrial process, providing a consistent and reliable production of baking soda.
- Sustainability: Solvay method generates fewer byproducts compared to other alternative methods, so it is an environment friendly process.
- Purity: This method yields to the production of high-quality baking soda, making it suitable for a range of applications such as baking and pharmaceuticals.
- Flexibility: The Solvay method can be adjusted easily to produce different amounts of baking soda to meet the varying needs of large-scale industrial production.
Limitations of Preparation of Baking Soda by Solvay Method
The limitations of preparation of baking soda by Solvay method are mentioned below:
- Ammonia Usage: The utilization of ammonia in the Solvay method poses environmental and safety challenges due to its impact and potential risks.
- Byproduct Generation: Solvay method produces calcium chloride as a byproduct, which requires proper disposal and management.
- Quality Control: Ensuring consistent quality becomes challenging in large scale production, which leads to the variations in the final product.
- Energy Intensive: This method is energy-intensive, influencing the overall environmental impact of the production of baking soda.
- Raw Material Dependence: Dependence on particular raw materials such as sodium chloride, makes the production process susceptible in their availability or cost.
History of Baking Soda
Baking soda has a rich history, which was first discovered by ancient Egyptians. Ancient Egyptians used a natural mineral called natron, which contains baking soda, for various purposes like cleaning. The Greeks and the Romans also used the same substance for cleaning and medicinal applications.
However, in the 19th century, the baking soda which we know today was emerged. In 1846, the two bakers from New York, John Dwight and Austin Church, established the first factory to produce baking soda. They developed a process to produce a reliable and high quality product. Since then, the popularity of baking soda has grown steadily and has been used for several purposes. It has a wide range of applications in baking and household activities.
Properties of Baking Soda
Baking Soda, which is chemically known as sodium bicarbonate has the following properties:
Physical Properties of Baking Soda
The physical properties of baking soda or sodium bicarbonate are as follows:
- State: It is a white crystalline powder at a room temperature.
- Solubility: Baking soda is soluble in water.
- Taste: It is slightly salty in taste.
- Odor: Baking soda is generally odorless.
- pH: It is alkaline with a pH around 9 when dissolved in water.
- Melting Point: Baking soda decomposes at around 50⁰C.
Chemical Properties of Baking Soda
The chemical properties of baking soda are mentioned below:
- Chemical Formula: NaHCO3
- Decomposition: On heating, baking decomposes to produce carbon dioxide, water and sodium carbonate. The chemical reaction occurs as follows:
2NaHCO3 + Heat → Na2CO3 + H2O + CO2
- Reactivity: Baking soda reacts with acids to produce carbon dioxide, causing leavening in baking.
- Buffering: It helps to maintain steady pH value in various solutions.
NaHCO3 + H → H2O + CO2 + Na+
- Neutralization: Baking Soda, which acts as a base, neutralizes acidic substances.
NaHCO3 + HCl → CO2 + H2O + NaCl
- Solubility: Baking soda is soluble in water, which is demonstrated by the following reaction:
NaHCO3 → Na+ + HCO3–
In water, baking soda dissociates into sodium ions (Na+) and bicarbonate ions (HCO3–), which contributes to its solubility.
- Weak Electrolyte: Baking soda, when dissolved in water, dissociates into sodium and bicarbonate ions
NaHCO3 → Na+ + HCO3–
This dissociation demonstrates its weak electrolyte behaviour in aqueous solutions.
Uses of Baking Soda
Baking Soda has various practical uses. Some of the common uses of baking soda are as follows:
- Baking: Baking soda acts as a leavening agent, which helps dough rise and makes baked goods soft.
- Fire Extinguisher: Baking soda has the ability to extinguish minor grease fires by suffocating the flames.
- Cleaning: Baking soda serves as a gentle cleaner that works effectively and helps eliminate odors.
- Deodorizing: Baking soda counters unpleasant smells in fridges, carpets, and shoes by neutralizing them.
- Personal Care: Baking soda is utilized for maintaining oral hygiene and used as a soothing bath additive.
- Heartburn Relief: Baking soda is used as an antacid to relieve heartburn and indigestion.
- Laundry: Baking soda is used to boost the detergent efficiency and to soften water during the washing process.
- pH Regulation: It maintains a stable pH level in swimming pools and adjusts acidity in cooking.
References
- https://www.healthline.com/nutrition/baking-soda-benefits-uses
- https://www.everydayhealth.com/diet-nutrition/diet/baking-soda-uses-benefits-side-effects-recipes-more/
- https://www.webmd.com/a-to-z-guides/baking-soda-do-dont
- https://www.britannica.com/science/sodium-bicarbonate
- https://www.chemicalsafetyfacts.org/chemicals/sodium-bicarbonate-baking-soda/
- https://byjus.com/chemistry/preparation-properties-and-uses-baking-soda/#:~:text=Baking%20soda%20is%20the%20common,of%20Na2CO3.
