Beryllium is a toxic bivalent element that is steel gray in color, strong, and light in weight and is mostly utilized as a hardening agent in alloys. Beryllium has one of the light metals’ highest melting points. It possesses high thermal conductivity, is nonmagnetic, resists attack by strong nitric acid, and resists oxidation when exposed to air at standard temperatures and pressures. Beryllium (Be) possesses a number of unique features that make it of theoretical and practical relevance. Its metal properties distinguish it from neighbor elements lithium, magnesium, and calcium.
Interesting Science Videos
History of Beryllium
- Although emeralds and beryl were known to ancient civilizations, Abbé Haüy identified them as the same material Be3Al2(SiO3)6 in 1798.
- Later that year, a French scientist named Louis-Nicholas Vauquelin found an undiscovered element in emeralds and beryl. He named it glucinium because it tasted sweet like glucose (sugar).
- Efforts to isolate the new element were ultimately successful in 1828 when two chemists, Friedrich Wölhler of Germany and A. Bussy of France, separately synthesized beryllium in a platinum crucible by reducing beryllium chloride (BeCl2) with potassium.
- Nowadays, beryllium is typically synthesized through chemical processes or electrolysis of a combination of molten beryllium chloride (BeCl2) and sodium chloride (NaCl) from the minerals beryl Be3Al2(SiO3)6 and bertrandite (4BeO2SiO2H2O).
When Be was discovered in the late 18th century, it was not recognized for its distinctive qualities and commercial potential until the 1920s.
Occurrence of Beryllium
Be is not a particularly rare element, and it may be found in over 50 minerals around the world in the form of silicates, carbonates, sulfates, and oxides.
Be is widely dispersed in the Earth’s crust and is predicted to occur to the level of 0.0002 percent in igneous rocks. It has a cosmic abundance of 20 on a scale where silicon, the benchmark, has a value of 1,000,000. The United States contains over 60% of the world’s beryllium and is by far the largest producer; other notable producers include China, Mozambique, and Brazil. Beryl crystals are typically less than 20 mm in size and include between 7% and 15% beryllium oxide, 17% to 19% Al2O3, and 60% to 70% SiO2.
There are about 30 recognized minerals containing Be, including
- beryl (Al2Be3Si6O18, a beryllium aluminum silicate)
- bertrandite (Be4Si2O7(OH)2, a beryllium silicate),
- phenakite (Be2SiO4), and
- chrysoberyl (BeAl2O4)
Isotopes of Beryllium
Beryllium (atomic number 4) contains twelve isotopes, but only three are usually measured: 7Be, 9Be, and 10Be. 9Be is a stable isotope found naturally in geological materials at μg g-1 levels.
Be is formed in stars, but the radioactive isotopes endure only a short time. Beryllium-9 is the only stable isotope present in primordial beryllium. Be is a single-nuclidic and single-isotopic element. Beryllium-10 is created in the atmosphere through cosmic ray spallation of oxygen.
Depending on the overall energy of the nucleus and its total angular momentum quantum number, many Be isotopes have numerous decay routes.
Elemental Properties
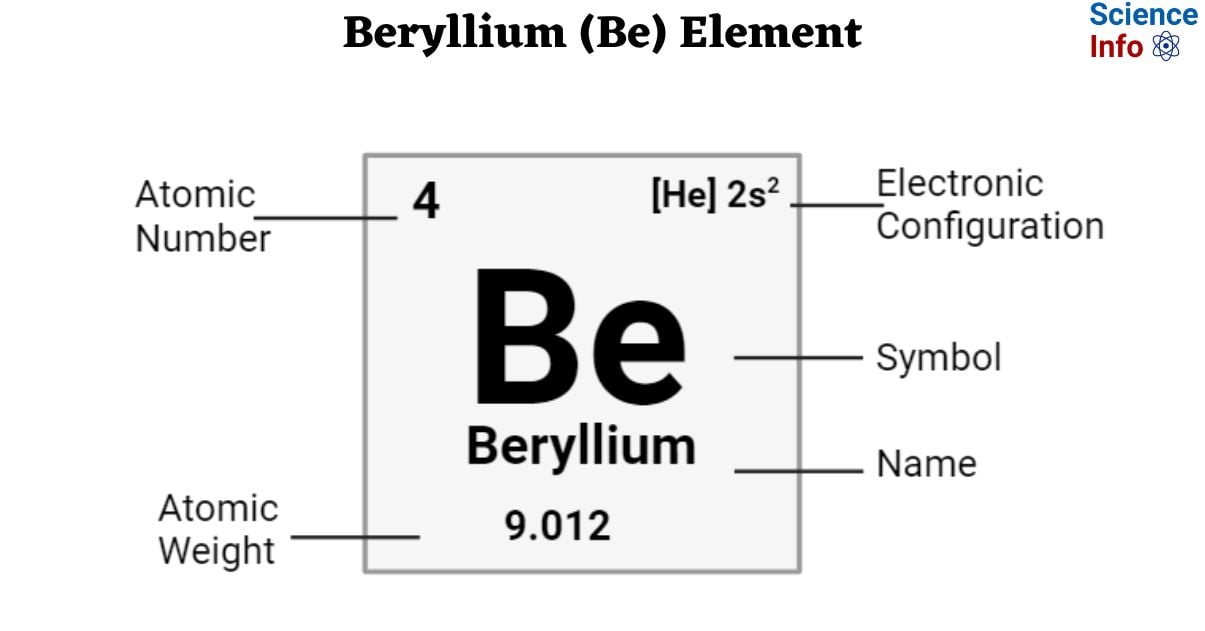
| Electronic Configuration | [He] 2s2 |
| Atomic Number | 4 |
| Atomic Weight | 9.012 atomic mass units |
| Group, Period, and Block | 2 (Alkaline Earth Metals), 2, s-block |
| Empirical Atomic Radius | 105 pm |
| Covalent radius | 96 ± 3 pm |
| Van der Waals radius | 153 pm |
| Electrons: | 4 |
| Protons: | 4 |
| Neutrons in the most abundant isotope: | 5 |
| Electron shells | 2, 2 |
Physical Properties of Beryllium
- Beryllium is a light, silver-gray metal that is comparatively soft and robust but brittle.
- Be has the greatest melting point of the light metals, melting at 1278 °C – far higher than Lithium (180°C), Sodium (98 °C), or Calcium (839 °C).
- Under normal circumstances, a thin layer of the hard oxide BeO forms on the surface of Be, protecting it from further attack by water or air.
- Be does not oxidize in air, even at 600°C, as a result of this BeO layer, and it is resistant to corrosion by strong nitric acid.
- Be is also nonmagnetic and has a high thermal conductivity.
- It is found in 30 distinct minerals, the most notable of which are bertrandite, phenakite, and beryl.
- Be content in the soil can be absorbed by plants if it is soluble.
| Melting Point | 1287°C, 2349°F, 1560 K |
| Boiling Point | 2468°C, 4474°F, 2741 K |
| Density | 1.85 g.cm-3 |
| Critical Temperature | 5205 K |
| Heat of Fusion | 12.2 kJ/mol |
| Heat of Vaporization | 292 kJ/mol |
| Molar Heat Capacity | 16.443 J/(mol·K) |
| Electronegativity | Pauling scale: 1.57 |
Chemical Properties of Beryllium
- Be produces hydrogen gas when it reacts with certain acids or with water.
- It interacts with oxygen to generate BeO (beryllium oxide) when exposed to air, but it resists oxidation at a specific standard pressure and temperature.
- Be chemical behavior is mostly determined by its tiny atomic and ionic radii. When connected to other atoms, it possesses very high ionization potentials and strong polarization, which is why all of its compounds are covalent. Its chemistry is comparable to that of aluminum, demonstrating a diagonal relationship.
- It dissolves well in non-oxidizing acids like HCl and dilute H2SO4, but not in nitric acid or water because it creates oxide. This is analogous to the behavior of aluminum metal. Be dissolves in alkali solutions as well.
- The hydrolysis of the [Be(H2O)4]2+ ion results in the acidic nature of solutions of Be salts, such as beryllium sulfate and beryllium nitrate.
Use and Application of Beryllium
- Be is utilized as an alloying agent.
- It has a wide temperature range thermal stability, high strength, non-magnetic characteristics, and improved resistance.
- Be is commonly used in the defense and aerospace industries when fused with copper to make alloys.
- Because Be is almost transparent to x-rays, unlike most metals, it is utilized in the radiation windows for x-ray tubes.
- Using copper or nickel, Be metal is alloyed to create springs, spot-welding electrodes, gyroscopes, and non-sparking tools
- It is utilized in nuclear operations and is also thought to have ceramic applications due to its high melting point.
- Moreover, they are dimensionally stable and exhibit non-magnetic characteristics throughout a wide temperature range, making them a good metal for alloys. For instance, Beralcast is an alloy made of Be and aluminum.
- Microwave ovens, high-speed computers, lasers, and other devices all employ Be compounds.
- Be is also employed in nuclear reactors as a shield, moderator, and neutron reflector and absorber.
Health and Environmental Effects of Beryllium
Health Effects
Beryllium is one of the most toxic chemicals we are aware of; it is not a necessary element for humans. It is a metal that can be extremely dangerous when inhaled by humans since it can damage the lungs and cause pneumonia.
Berylliosis: The most well-known side effect of beryllium is berylliosis, a serious lung condition that can potentially harm other organs like the heart. About 20% of all instances result in death from this illness. The condition known as berylliosis is brought on by breathing Be while work. The most vulnerable individuals to this illness are those with compromised immune systems.
Beryllium Sensitization: Activation of the body’s immunological response to beryllium is known as beryllium sensitization. When Be dust, fume, mist, or solutions are inhaled or come into contact with the skin, beryllium sensitization may occur. While sensitization may not be accompanied by any clinical signs, a person who has undergone beryllium inhalation exposure runs the risk of acquiring CBD.
In addition to causing berylliosis and CBD, Be can also raise the risk of cancer growth and Genetic deterioration.
Chronic beryllium disease (CBD): Inhaling airborne Be after developing a beryllium sensitivity results in chronic granulomatous lung disease known as chronic beryllium disease (CBD). Breathlessness, unexplained coughing, exhaustion, weight loss, fever, and night sweats are some of the symptoms of CBD that are frequently seen.
When CBD becomes a chronic obstructive pulmonary condition, it can lower life expectancy and lower quality of life (for additional information see section on Treatment below).
CBD and pulmonary sarcoidosis, a granulomatous lung disease with no known cause or origin, share similar signs and symptoms. Without a proper diagnosis, it might be challenging to tell CBD from sarcoidosis.
Allergic Reactions: Those who are particularly allergic to Be may also experience allergic responses.
Acute Beryllium Disease (ABD): It is a form of chemical pneumonia with a quick start that is brought on by breathing in Be concentrations that are high in the air. In 10% of instances, ABD can be deadly and is typically linked to exposure to Be levels at or above 100 g/m3.
Environmental Effects
- As a result of both natural and man-made processes, Be is released into the air, water, and soil. It is present in the environment on a tiny scale naturally. By making metal and burning coal and oil, humans add Be to the environment.
- Be is present in the atmosphere as tiny dust particles. It enters streams as a result of soil and rock degradation. Both industrial emissions and wastewater disposals will increase the amount of Be in the air and water, respectively.
- Normally, it sinks in sediment. Little amounts of Be are a chemical element that naturally occur in soils, however human activities have also raised these Be levels. It is unlikely that Be will sift through groundwater and penetrate farther into the soil.
- It will react chemically with water, making it insoluble. This is advantageous since Be in its water-insoluble form can harm organisms far less than Be in its water-soluble form.
- Fish bodies won’t become contaminated with Be. Yet, some fruits and vegetables, such pears and kidney beans, may have considerable Be content. Fortunately, the majority of animals quickly excrete Be through urine and feces, preventing these amounts from entering animals that eat them.
Toxicity, Safety and Prevention
There are no special health hazards associated with Be metal in its solid state or when used in finished goods. When melting, casting, handling dross, pickling, chemical cleaning, heat treating, abrasive cutting, welding, grinding, sanding, polishing, milling, crushing, or otherwise heating or abrading the surface of this material in a way that generates particulate, exposure to the substances listed can happen by inhalation, ingestion, and skin contact.
Exposure can also happen while performing repairs or maintenance on contaminated equipment, such as when rebuilding a furnace, maintaining or repairing air cleaning equipment, renovating a building, welding, etc. During routine hand-to-face activities such rubbing the nose or eyes, sneezing, coughing, etc., particulate deposition on hands, gloves, and clothing can be transferred to the breathing zone and inhaled.
Major routes of entry: inhalation, eye and skin contact.
Exposure to the eyes: It can occur through direct contact with airborne particulates or through contact with contaminated hands or clothing. Particulate can cause irritation or mechanical harm to the eyes.
Skin: Particle that becomes stuck under the skin can cause hypersensitivity and skin sores.
Inhalation: May irritate the mucous membranes in the nose, throat, lungs, and other areas. The Be present in this product is not known to have any immediate negative impact on health. Some people who breathe in beryllium-containing particles may develop Chronic Beryllium Disease (CBD), a dangerous, persistent lung condition.
Long-term exposure: Chronic beryllium disease (CBD), a dangerous, long-lasting lung condition, may be brought on in some people by inhaling particles containing Be.
Safety Measures
- After making eye contact, immediately rinse your eyes thoroughly with water for at least 15 minutes while occasionally elevating your top and lower eyelids. Seek medical help right away.
- Remove all contaminated clothing, wash it, and then dry it. Wash skin cuts and wounds thoroughly to get rid of any granular material. For wounds that cannot be properly cleaned, need medical help. Before starting work, treat skin cuts and wounds using common first aid procedures such washing, disinfecting, and covering to stop infection and contamination. If you are constantly irritated, get medical attention. It is necessary to remove any material unintentionally implanted or lodged under the skin.
- Breathing discomfort brought on by particle inhalation necessitates a swift transfer to fresh air. If you have breathing problems, you could need oxygen. Use artificial respiration if breathing has ceased, then seek medical attention.
- Most significant acute and delayed signs and symptoms: could result in an allergic skin reaction. allergic respiratory response could occur. Chronic consequences may result from prolonged exposure.
Handling and Storage
- Particles may enter the body through cuts, abrasions, or other wounds on the skin’s surface. Care to be taken in handling and storage. When handling parts with loose surface particles or sharp edges, put on gloves.
- Keep at a cool temperature in a dry location.
- The best way to reduce exposure to airborne particulates is to use local exhaust ventilation or other engineering measures whenever practical. When a ventilation system is used, exhaust inlets must be placed as close as feasible to the source of airborne production.
- Avoid using tools like man-cooling fans to alter the airflow near a local exhaust intake.
- To make sure ventilation equipment is operating properly, inspect it frequently.
- All users should receive instruction on how to utilize and operate the ventilation system. Use trained experts to create and install ventilation systems.
Fun Facts of Beryllium
- With 170 metric tons of beryllium mined in 2021, the United States is the Be producer with the highest production rates worldwide. With 70 metric tons mined in 2021, China is the second-largest producer of Be worldwide.
- The neutron was discovered in 1932 by English physicist James Chadwick while working with Be.
- Mirrors constructed of Be are used in the James Webb Space Telescope, which was sent into orbit in December 2021 and is roughly one million kilometers from Earth.
- The European Commission lists beryllium as one of the 20 critical raw materials for the European Union.
- Due to its chemical resemblance to magnesium, Be has the ability to replace magnesium in human body enzymes. This frequently results in the enzymes malfunctioning, which may have extremely negative effects.
- For better castability and bond strength, some dental materials (such the frameworks for crowns, bridges, and partial dentures) contain beryllium.
- Beryllium frequently has a significant function in nuclear facilities. In fusion reactors, it acts as a plasma-facing material and as a neutron reflector in fission reactors.
References
- Walsh, Kenneth A. Beryllium Chemistry and Processing. ASM Intl (2009).
US Geological Survey. Brian W. Jaskula. - Beryllium Science & Technology Association. About Beryllium.
- Vulcan, Tom. Beryllium Basics: Building On Strength As A Critical & Strategic Metal. Minerals Yearbook 2011. Beryllium.
- https://chemistrytalk.org/beryllium-element/
- https://www.osha.gov/beryllium/health-effects
- https://www.britannica.com/science/beryllium#ref8230
- https://education.jlab.org/itselemental/ele004.html
- https://www.lenntech.com/periodic/elements/be.html
- Safety Data Sheet: Product No. 1655, 1655-B, 1656, Beryllium.

