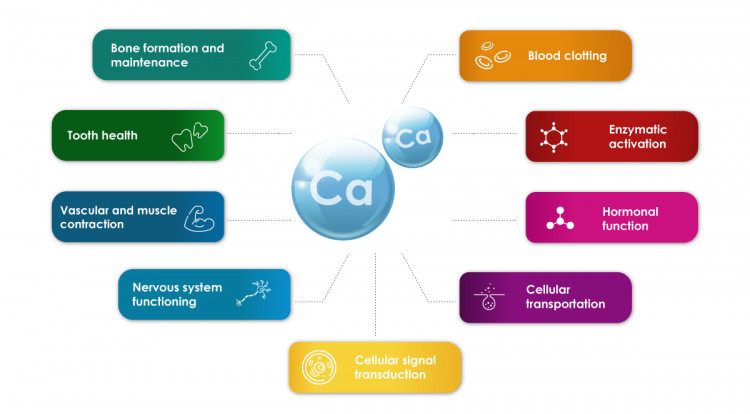
The biological function of calcium is immense in our body. The human body contains the highest concentration of inorganic calcium, which is vital to numerous physiological functions. For example, Ca2+ functions as a messenger and mediator for cardiac, skeletal, and smooth muscle contractions, among its many other intra- and extracellular physiological tasks. When it comes to the endocrine, neurological, and renal components of blood pressure regulation, calcium ions play a crucial role in several biological processes that are essential to life.

Calcium is a soft grey alkaline earth metal with the atomic number 20 and symbol Ca. There are four stable isotopes of calcium (40Ca, 42Ca–44Ca), and when the metal combines with water, calcium hydroxide and hydrogen are produced.
2Ca + 2H2O → 2CaOH + H2
Calcium salts are ubiquitous in our daily lives, with examples ranging from limestone, cement, and lime scale, to fossils, all containing the Ca2+ ion. These salts have diverse applications, extending from insecticides to clinical uses.
- Calcium arsenate (Ca3(AsO4)2) is highly toxic and serves as an insecticide. In clinical settings, calcium carbonate (CaCO3) is present in antacids, though excessive consumption poses risks.
- Calcium chloride (CaCl2) is utilized for ice removal, dust control on dirt roads, concrete conditioning, and as an additive in canned tomatoes.
- Calcium cyclamate (Ca(C6H11NHSO4)2) acts as a sweetening agent, while calcium gluconate (Ca(C6H11O7)2) is a food additive in vitamin pills.
- Calcium hypochlorite (Ca(OCl)2) is found in swimming pool disinfectants, bleaching agents, deodorants, and fungicides.
- Calcium permanganate (Ca(MnO4)2) is employed in textile production, water sterilization, and dental procedures.
- Calcium phosphate (Ca3(PO4)2) serves as a supplement for animal feed, and a fertilizer, and is used in glass manufacturing and dental products.
- Lastly, calcium sulfate (CaSO4⋅2H2O) is commonly found in blackboard chalk.
Interesting Science Videos
Biological Function of Calcium
The human body uses calcium ions for several neurological and endocrinological functions. As a cellular messenger, calcium has a significant intra-versus extracellular gradient (1: 10,000), which is extensively influenced by hormones. To keep cells receptive to various extracellular stimuli, this gradient is required. In addition, calcium ions have a role in the development of teeth and bones, which serve as calcium ion reservoirs.
An average adult body typically holds approximately 1000 grams of calcium, with nearly 99% of it located extracellularly, primarily stored in bones and teeth. Bones function as a dynamic reservoir for Ca2+. The remaining 1% of Ca2+ is distributed in the extracellular space, including plasma, lymph, and extracellular water. Precise regulation of intra and extracellular Ca2+ concentrations is crucial for numerous physiological processes, underscoring the importance of stringent control.
- The regulation of calcium ions occurs in the gastrointestinal tract, skeleton, and kidneys.
- Typically, Ca2+ homeostasis is maintained in a state of equilibrium, where the intake of calcium into the body equals the amount excreted.
- Hormones unrelated to Ca2+ levels, known as noncalciotropic hormones—such as sex hormones and growth factors—play a role in regulating calcium ion levels.
- On the other hand, calciotropic hormones, like parathyroid hormone (PTH), are directly linked to Ca2+ regulation.
- PTH governs the serum plasma Ca2+ levels by overseeing the reabsorption of Ca2+ in the nephron, promoting the absorption of Ca2+ from the gastrointestinal tract, and releasing Ca2+ from bones, serving as a calcium reservoir.
- About half of our bones are composed of modified hydroxylapatite, also commonly referred to as hydroxyapatite or bone mineral.
- The mineral calcium apatite, whose formula is typically written as Ca10(PO4)6(OH)2, exists naturally as hydroxylapatite.
- Teeth also contain hydroxylapatite modifications, and a chemically similar material is frequently employed as a filler to replace lost bone. However, even if these chemicals have similar or equal chemical components, the way the body reacts to them might differ greatly.
How does dietary calcium intake affect our lives?
It is thought that consuming the right amount of calcium through food might stave off chronic illnesses. The average adult’s daily calcium consumption during the Stone Age was between 2000 to 3000 mg Ca2+, whereas it is currently only 600 mg. This indicates that there is a constant calcium shortage in our bodies, which is thought to have connections to many chronic illnesses like high blood pressure, colon cancer, and fragile bones.
Calcium (Ca2+) is a vital nutrient, and the necessary intake varies at different life stages. There are three recognized life stages during which the human body demands an elevated level of Ca2+.
- The initial stage is childhood and adolescence, spanning from birth to approximately 18 years, as bones undergo formation and growth until reaching peak strength.
- Another critical period is pregnancy and lactation, where the body requires increased Ca2+ levels.
- An infant accrues about 30 g of Ca2+ during gestation and an additional 160–300 mg/day during lactation.
- Aging constitutes the third phase in life when augmented calcium intake is essential. This requirement is linked to various changes in calcium metabolism observed in the elderly.
Effects of Calcium deficiency
Osteoporosis
- Osteoporosis is commonly linked to a deficiency in calcium, but the importance of sufficient calcium intake extends beyond treating bone loss; it should be regarded as a fundamental strategy for maintaining overall health as one age.
- The bones, acting as a reservoir, contain 99% of Ca2+.
- Osteoporosis stands out as a primary factor contributing to bone fractures, particularly in postmenopausal women.
- Hormones play a crucial role in regulating calcium uptake and plasma concentrations. However, a definitive and direct correlation between Ca2+ intake and bone health has not been conclusively established thus far.
- The prevailing belief is that maintaining optimal bone density requires a high Ca2+ concentration and vitamin D level during the initial three decades of life. These factors also influence the rate of bone loss associated with the aging process.
Hypertension
- Research supports the idea that taking supplements of calcium might lower blood pressure, with a greater benefit for salt-dependent hypertension.
- A key component of maintaining blood pressure homeostasis is controlling the metabolism of calcium within cells.
- It is thought that smooth muscle vasoconstrictor tone increases with cytosolic-free calcium ion levels. This, in turn, influences sympathetic nervous system activity and blood pressure. However, research does not support treating patients with moderate hypertension exclusively with calcium supplements.
- There is a hypothesis that suggests a connection between calcium intake in the diet and human weight control. It has been suggested that a diet high in calcium and low in calories can help combat obesity and improve energy metabolism.
- Depending on age, the recommended daily intake of Ca2+ should be approximately 1200 mg.
- Existing data suggests that consuming more calcium may significantly lower the chance of being overweight; however, large-scale, prospective clinical trials over an extended period of time are required to validate or further elucidate this relationship.
Renal osteodystrophy
- Patients suffering from chronic or end-stage renal failure may have renal osteodystrophy, also known as renal bone disease, which is a lack of bone mineralization.
- The prohormone calcidiol in the liver and the active form of vitamin D, calcitriol, in the kidney are the normal sites of activation of vitamin D. A hydroxylation process serves as the basis for both activation phases. Calcidol, the liver’s 25-position pro-vitamin D hydroxylation, and calcitriol, the kidney’s 1α-position pro-vitamin D hydroxylation, occur simultaneously. Calcitriol facilitates the body’s uptake of dietary Ca2+.
- Ca2+ concentration in blood plasma is lowered in individuals with renal failure due to a reduced activation to calcitriol. In addition, the dysfunction of the kidneys raises the level of plasma phosphate. As a result of the phosphate complexing the free Ca2+, the blood’s concentration of free Ca2+ is further decreased.
- When plasma Ca2+ levels are low, the pituitary gland detects this and releases PTH. As mentioned earlier, PTH stimulates the release of Ca2+ from the bones and enhances the re-absorption of Ca2+ in the nephron and absorption in the gut. Consequently, this causes the bone structure to deteriorate.
Phosphate binders can be used to treat patients to prevent the intestines from absorbing too much phosphate. Additionally useful in clearing the blood of excess phosphate is dialysis. In addition, the patient may receive supplements including calcium and synthetic calcitriol.
Kidney stones
- Approximately 20–40% of kidney stones are linked to an increased level of Ca2+ in urine.
- Traditional wisdom has suggested that a low dietary calcium intake is the optimal approach to prevent recurring kidney stones. However, recent studies involving individuals prone to calcium oxalate stones revealed that a low calcium diet does not deter the formation of kidney stones.
- Surprisingly, it was discovered that a higher calcium intake, reaching around 1200 mg/day, led to a substantial 50% reduction in the recurrence of kidney stones.
- The theory is that restricting calcium results in heightened absorption and excretion of oxalate in the urine, thus fostering the development of calcium oxalate stones.
- Currently, the research shows that calcium supplementation is not linked to kidney stone formation in healthy individuals.
Clinical application of Calcium
Typically, calcium supplements are only needed in cases where dietary Ca2+ consumption is inadequate. Nutritional needs vary depending on age and situation; children, pregnant women, and the elderly, who have reduced absorption, for example, have higher needs. A gradual intravenous infusion of 10% calcium gluconate has been advised in cases of severe acute hypocalcaemia. Remember that you need to closely monitor the plasma Ca2+ level and any changes to the electrocardiogram (ECG).
- Many calcium salts, such as calcium carbonate, calcium chloride, calcium phosphate, calcium lactate, calcium aspartate, and calcium gluconate, are employed in clinical settings. The most popular and least-priced calcium supplement is calcium carbonate. Because of the way stomach HCl reacts with carbonate to produce CO2, it can be hard to digest and induce gas in certain people.
- It is advised to take calcium carbonate with food, and the pH levels affect how quickly the gut absorbs the calcium carbonate. Along with it, using magnesium salts can help avoid constipation. Since calcium carbonate contains 40% Ca2+, 1000 mg of salt will have approximately 400 mg of Ca2+. Labels frequently do not list the amount of calcium carbonate in a tablet; only the amount of Ca2+ is listed.
- Compared to calcium carbonate, calcium citrate is easier to digest, less prone to induce gas and constipation, and more readily absorbed (bioavailability is 2.5 times higher). On an empty stomach, calcium citrate is easier to absorb than calcium carbonate and can be taken without meals. Additionally, it’s thought to have a smaller role in kidney stone formation. About 24% of calcium citrate is composed of Ca2+, meaning that 240 mg of Ca2+ is present in 1000 mg of calcium citrate. It is a more expensive treatment alternative than calcium carbonate due to its lower Ca2+ content and higher cost, but its marginally different application field can explain this.
- Although calcium lactate is often more expensive, its characteristics are similar to those of calcium carbonate. Comparing calcium lactate to calcium carbonate, for example, there is practically less Ca2+ per gram salt. Because calcium lactate only contains 18% Ca2+, it is a less “concentrated” salt.
- In addition to being used as a calcium supplement, calcium gluconate is also utilized to treat hyperkalaemia—defined as K+ plasma levels greater than 6.5 mmol/l—urgently.
- When there are ECG abnormalities and hyperkaleamia, treatment should be started right once. An intravenous 10% calcium gluconate solution is advised. While giving the calcium solution does not lower the plasma K+ level, it does momentarily lessen the harmful effects of hyperkalaemia by protecting cardiac excitability. At only about 9%, calcium gluconate has the least amount of Ca2+ per dose. This indicates that only 90 mg of the 1000 mg of calcium gluconate are real Ca2+.
Side Effects of Calcium
Several extensive, long-term studies have indicated the safety of a daily intake ranging from 1000 to 2500 mg of calcium salts. Adverse effects are generally observed only at relatively high doses, leading to gastrointestinal issues like constipation and bloating, and in extreme cases, arrhythmia.
- Gastrointestinal problems tend to self-adjust over time.
- Calcium salts are typically better absorbed in an acidic environment, so individuals with low stomach acid production or elderly patients on high doses of antiulcer medication may encounter absorption issues, and it is advisable to take calcium supplements with meals in such cases.
- It’s crucial to recognize that calcium ions can impede the absorption of certain medications, including antibiotics. For instance, tetracycline and quinolone antibiotics can chelate Ca2+ ions, forming complexes that are no longer absorbable.
- Therefore, it is recommended not to take calcium supplements and antibiotics simultaneously. Patients are generally advised to take antibiotics either one hour before or two hours after a meal.
Video on Biological Function of Calcium
References
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. PMID: 25182228 pubmed.ncbi.nlm.nih.gov/25182228.
- Going Crazy over Calcium” in the Health and Fitness section of Time, February 23, 1987, p. 49, or C. Garland et al., The Calcium Diet, Penguin Books, 1990
- R. J. P. Williams, in Calcium in Biological Systems, Cambridge Univ. Press, 1976, p. 1.
- https://medlineplus.gov/ency/article/002412.htm#:~:text=Function&text=Calcium%20is%20one%20of%20the,lifetime%20can%20help%20prevent%20osteoporosis.
- https://link.springer.com/referenceworkentry/10.1007/978-1-4614-1533-6_66