The biological function of magnesium (Mg), an alkaline earth metal with a silvery-white color, is of high importance in our body. The body uses magnesium (Mg2+) for many processes, including bone development, neuromuscular function, signaling pathways, energy storage and transfer, glucose, lipid and protein metabolism, DNA and RNA stability, and cell proliferation. Mg2+ is involved in almost all major metabolic and biochemical processes that occur within the cell.
Magnesium is shielded by a thin oxide layer that is both extremely difficult to remove and eliminates the need for oxygen-free storage. When it comes to water, magnesium reacts somewhat more slowly than calcium, an alkaline element that is nearby on Earth. Magnesium is a highly flammable metal that burns with a distinctive bright white blaze when ignited. Magnesium has three stable isotopes: 24magnesium (79% occurrence), 25 magnesium, and 26 magnesium. Radioactive 28 mg has a half-life of 21 hours.
When consumed in significant quantities, the majority of magnesium salts dissolve in water and have laxative properties for the body. The flavor of aqueous magnesium ions is sour. The resulting suspension, known as milk of magnesia and frequently employed as an antacid due to its mild base properties, is made from magnesium hydroxide i.e. Mg(OH)2, which is only soluble in a small amount of water. Large-scale magnesium extraction is accomplished by the ferrosilicon method or Down’s cell, both of which use seawater as the primary source. Because of their so-called diagonal relationship, Mg2+ and Li+ exhibit comparable characteristics and biological activity.
Interesting Science Videos
Biological Function of Magnesium
Magnesium (Mg2+) is a vital ion within the human body, playing a pivotal role in various enzymatic processes.
- It is an indispensable component in numerous cellular functions, serving as a signaling molecule and participating in nucleic acid biochemistry, including the regulation of ATP (adenosine triphosphate), DNA, RNA, and related processes. Specifically, the biological activity of ATP requires coordination with a magnesium ion. Additionally, Mg2+ contributes to the stabilization of DNA and RNA structures, evident in their elevated melting points.
- Magnesium ions (Mg2+) serve as the redox-active center in chlorophyll, playing a vital role in the photosynthesis process and the associated carbon fixation in green plants. As a result, magnesium-rich sources include green vegetables, milk, whole grains, and nuts. However, it is important to note that since most magnesium salts are water-soluble, processed vegetables—often cooked in water—tend to have lower magnesium ion content.
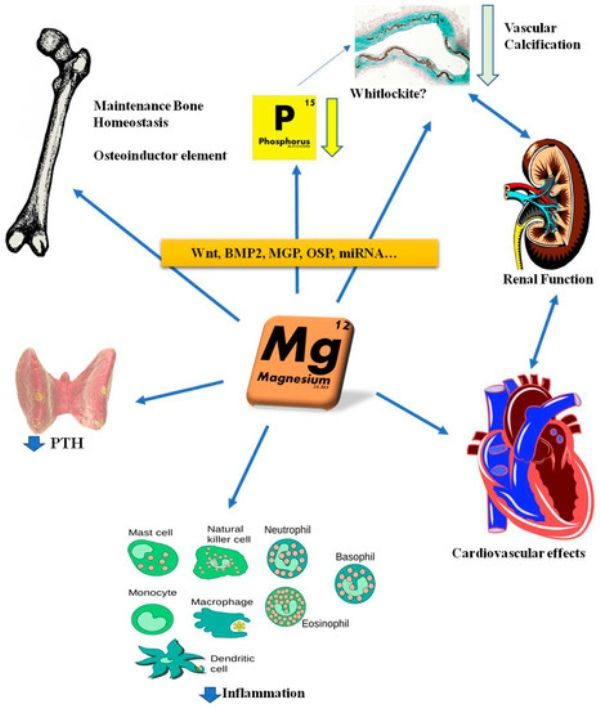
- Mg2+ is the second most common ion in the interstitial fluid and the fourth most common cation in the human body.
- Magnesium (Mg2+) is a necessary co-factor for over 300 cellular enzyme activities. The human body has approximately 24 g of magnesium ions on average, half of which are found in muscles and soft tissue and half of which are integrated into bones.
- The kidneys are the main organ for the excretion of Mg2+, with the ilium and colon absorbing the majority of it. The glomerulus filters the Mg2+, which is then reabsorbed at the proximal tubule (10–15%), the thick section of the ascending limb of the loop of Henle (60–70%), and the distal tubule (10–15%).
- However, magnesium salts are generally poorly absorbed from the gastrointestinal (GI) tract, making them commonly utilized as osmotic laxatives. Plasma levels of magnesium ions are regulated by the kidneys, and in cases of renal failure, elevated Mg2+ levels may accumulate. This condition, known as hypermagnesia, can lead to muscle weakness and arrhythmia, although it is an infrequent occurrence.
- On the other hand, hypomagnesia, characterized by low magnesium levels in blood plasma, may result from GI tract losses, such as excessive diarrhea. Imbalances in magnesium levels can also stem from alcoholism or be induced by specific drug treatments.
- Hypomagnesia is often accompanied by hypocalcemia (low calcium ion plasma levels), as well as hypokalemia and hyponatremia.
Clinical Applications of Magnesium
Several illnesses, including hypo- and hypermagnesaemia, can be caused by abnormalities in the magnesium ions. Additionally, preparations of magnesium ions are used as antacids, usually in conjunction with salts based on aluminum. Magnesium salts are also used to treat eclampsia, a potentially fatal hypertension condition that affects pregnant women, and arrhythmia, or irregular heartbeat.
- A period of five days or more with plasma serum Mg2+ levels of less than 0.5–1 mmol/kg is linked to symptomatic hypomagnesaemia.
- Initially, Mg2+ ions are administered intravenously (IV) or intramuscularly (IM) as magnesium sulfate (MgSO4) injections, the latter of which can be somewhat painful. Additionally, particularly serious arrhythmias—disorders of the heart beat, or pulse—can be treated with mgSO4 in an emergency. It is often administered intravenously as a single dosage or with a single repeat in an emergency.
- Because the kidneys are the organs that eliminate Mg2+, it is important to keep an eye on the concentration of magnesium in the plasma and to lower the dosage in patients who have impaired kidney function. The patient may also get magnesium ions orally, for instance in the form of magnesium glycerophosphate tablets.
- Due to its laxative qualities, magnesium hydroxide [Mg(OH)2] is a common constituent in antacids and the primary component in “milk of magnesia.” Because of the low aqueous solubility of Mg(OH)2, the suspension of Mg(OH)2 in water, known as the “milk of magnesia,” resembles milk. It is administered to the patient for heartburn and dyspepsia and is regarded as a strong electrolyte and weak base. As an antacid, the alkaline suspension acts by neutralizing any excess stomach acid. Furthermore, it promotes bowel movement because the osmotic action of the magnesium ions raises the water content in the intestines, softening any feces that may be present.
- Additionally, magnesium trisilicate (Mg2Si3O8) can be found in antacid formulations, particularly those intended to treat peptic ulcers. The mechanism of action involves raising the pH of the gastric juice and causing a precipitate of colloidal silica to develop, protecting the mucosa. The majority of antacids are made up of a combination of magnesium and/or calcium preparations and aluminum hydroxide [Al(OH)3]. As a result, the mechanism of action will be covered in more detail in the chapter on medications containing aluminum.
Unfortunately, when magnesium salts are ingested orally, they may interact with concurrently administered drugs. For instance, magnesium trisilicate can impede the absorption of iron products, certain antibiotics (such as Nitrofurantoin), and antimalarial drugs (such as Proguanil). Preparations containing magnesium salts, which are components of antacids, are not recommended to be ingested concurrently with various drugs like ACE inhibitors, aspirin, and penicillamine. Typically, antacids diminish the absorption of co-administered medications. Therefore, before commencing antacid treatment, it is crucial to gather the complete medical history of the patient and evaluate potential interactions.
Video on Importance of Magnesium
References
- Diana Fiorentini, Concettina Cappadone, Giovanna Farruggia,* and Cecilia Prata; Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. 10.3390/nu13041136
- Robert H. Kretsinger, Magnesium in Biological Systems
- https://byjus.com/chemistry/biological-importance-of-calcium-and-magnesium/