The Brønsted-Lowry Acid theory is an extension of the Arrhenius theory of acids and bases. The Arrhenius theory states that in an aqueous solution, an acid will raise the concentration of H+ ions whereas a base will raise the concentration of OH– ions. The Arrhenius theory has a restriction in that it only detects the reaction of an acid and a base in an aqueous medium.
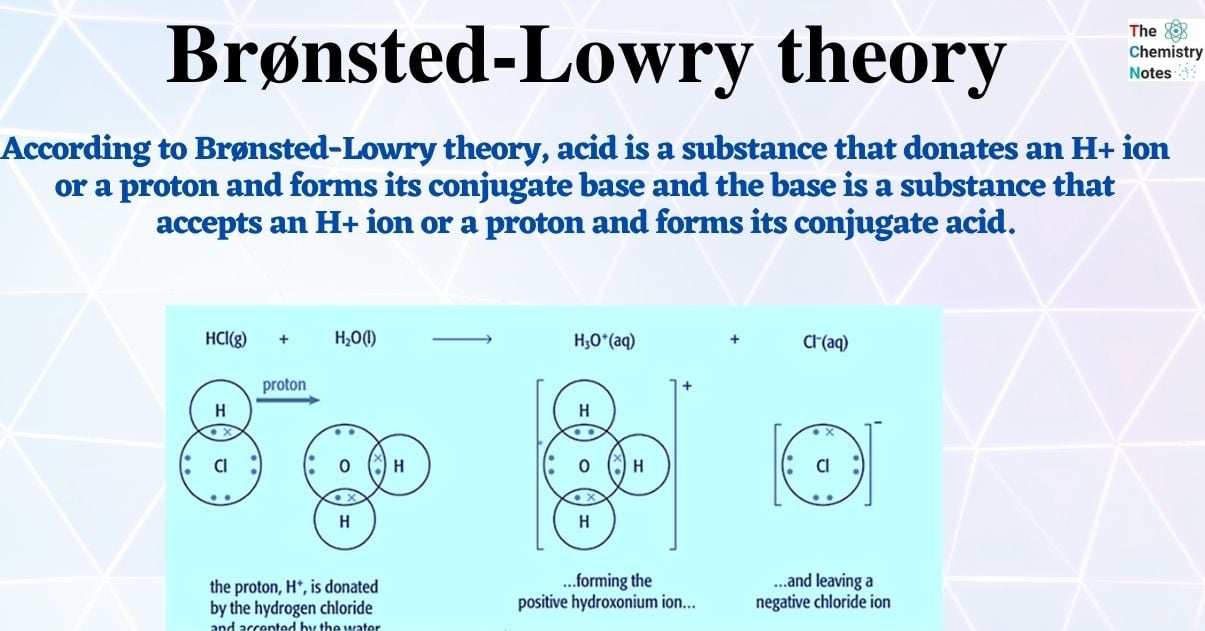
According to Brønsted-Lowry theory, acid is a substance that donates an H+ ion or a proton and forms its conjugate base and the base is a substance that accepts an H+ ion or a proton and forms its conjugate acid.
The Brønsted-Lowry hypothesis is a broader definition that can describe acid-base behavior under a larger range of situations. A Brønsted-Lowry acid-base reaction happens whenever a proton is transported from one reactant to the other, regardless of the solvent.
This does not preclude Arrhenius; rather, it broadens the concept of acidity to include non-aqueous systems.
According to the Brønsted-Lowry theory, bases are proton acceptors while acids are proton givers.
Since a proton is simply an H+ ion, hydrogen is a component of all Brønsted Lowry acids.
Acids and bases exist as conjugate pairs. The conjugate base is created by the acid when it gives a proton. A base transform into its conjugate acid when it takes a proton.
Compounds that work as both acids and bases are amphoteric.
Interesting Science Videos
The Brønsted–Lowry theory of acids and bases
This definition is based on the notion that a proton is transferred from an acid to a base during an acid-base reaction (a proton is a hydrogen ion, H+).
A proton donor is a Brønsted-Lowry acid.
A proton acceptor is a Brønsted-Lowry base.
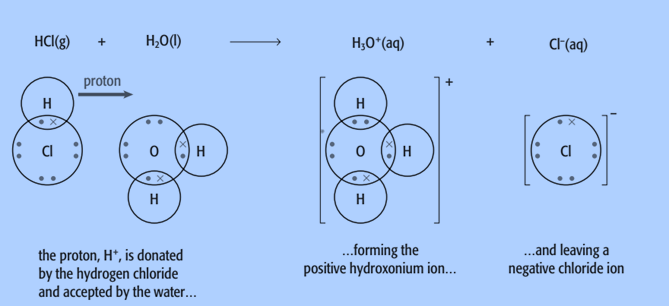
Hydrogen chloride gas dissolves in water to produce hydrochloric acid, which then reacts to produce hydronium ions, H3O+, and chloride ions.
Hydrogen chloride gas dissolves in water to produce hydrochloric acid, which then reacts to produce hydronium ions, H3O+, and chloride ions. You can observe that the reaction involves the presence of water.
HCl (g) + H2O (l) → H3O+ (aq) + Cl– (aq)
Hydrochloric acid is an acid because it donates a proton to water. Water is thus serving as a Brønsted-Lowry base, according to this. A proton is being accepted by the water.
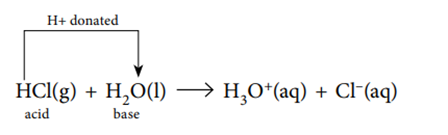
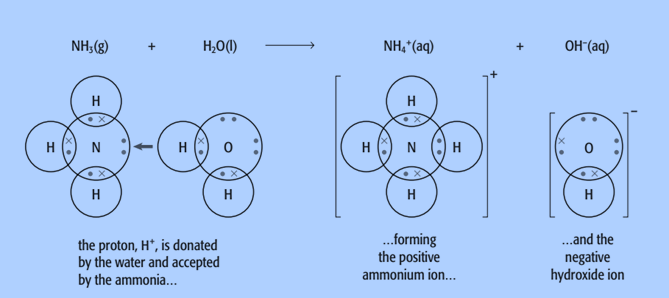
Water can also act as an acid. When ammonia reacts with water, it accepts a proton from the water and becomes an NH4+ ion.

Aqueous solutions are not required for the formation of Brønsted-Lowry acids and bases. When chloric (VII) acid (HClO4) combines with ethanoic acid (CH3COOH) in an inert solvent, for example, the following equilibrium is established.
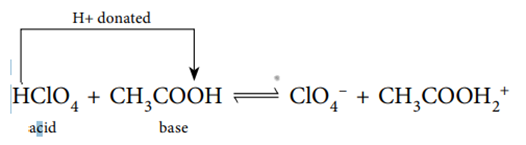
Since it is giving a proton to CH3COOH in this reaction, HClO4 is the acid. The fact that CH3COOH is a proton acceptor makes it the base. However, in the case of ammonia, the position of equilibrium favors the reactants. This can be demonstrated using equations. As an example,
NH3 (g) + H2O (l) ⇌ NH4 + (aq) + OH– (aq)
This reaction does not go to completion.
Conjugate acids and conjugate bases
In an equilibrium reaction, products are converted to reactants at the same rate as reactants are converted to products. In terms of the Brønsted-Lowry theory of acids and bases, the reverse reaction can also be considered.
Consider the reaction:
NH3 (g) + H2O (l) ⇌ NH4 + (aq) + OH– (aq)
In the reverse reaction, the NH4 + ion donates a proton to the OH– ion. So NH4 + acts as an acid and OH– acts as a base.
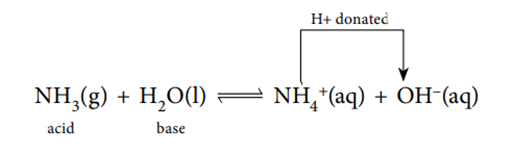
If a reactant is linked to a product by the transfer of a proton we call this pair a conjugate pair. Consider the following reaction
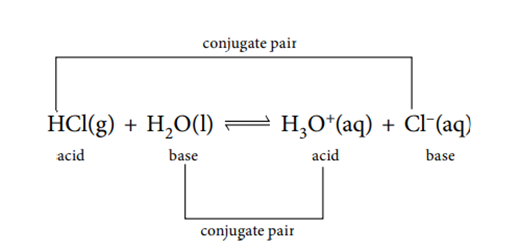
Looking at the forward reaction:
- Cl– is the conjugate base of the acid HCl
- H3O+ is the conjugate acid of the base H2O
Looking at the reverse reaction:
- HCl is the conjugate acid of the base Cl–
- H2O is the conjugate base of the acid H3O+.
In a conjugate pair, the acid has one proton more. The conjugate pairs for the equilibrium between ammonia and water to form ammonium ions and hydroxide ions are:
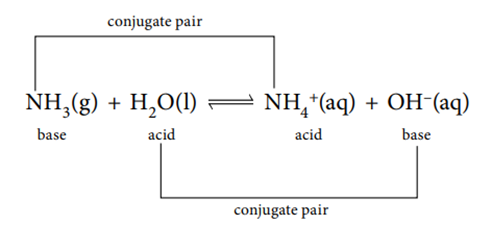
The idea of conjugate acids and bases is sometimes called the acid–1 base–1, acid–2 base–2 concept.
Brønsted – Lowry Strong and Weak, Acids and Bases
An acid or a base might be strong or weak.
In its solvent, which is usually water, a strong acid or base completely dissociates into its ion. A strong acid completely changes into its conjugate base, while a strong base completely converts into its conjugate acid. Strong acid’s conjugate base is extremely weak. A strong base’s conjugate acid is extremely weak.
Hydrochloric acid (HCl), nitric acid (HNO3), sulfuric acid (H2SO4), and hydrobromic acid (HBr) are all examples of strong Brønsted Lowry acids
Sodium hydroxide (NaOH), potassium hydroxide (KOH), lithium hydroxide (LiOH), and calcium hydroxide (Ca (OH2) are all examples of strong bases.
Incomplete dissociation of a weak acid or base results in an equilibrium condition in which both the weak acid and its conjugate base or weak base and its conjugate acid remain in solution.
Phosphoric acid (H3PO4), nitrous acid (HNO2), and acetic acid are examples of mild Brønsted Lowry acids (CH3COOH).
Ammonia (NH3), copper hydroxide (Cu (OH)2), and methylamine (CH3NH2) are examples of weak bases.
Remember that water is amphoteric, meaning it can function as an acid in some reactions and as a base in others. When a strong acid is dissolved in water, the water functions as a base. When a strong base is dissolved in water, the water becomes an acid.
For example:
HCl (aq) + H2O (l) → H3O + (aq) + Cl– (aq)
The conjugate pairs are as follows:
- HCl (acid) and Cl– (conjugate base)
- H2O (base) and H3O+ (conjugate acid)
NaOH (s) + H2O (l) → Na+ (aq) + OH– (aq)
The conjugate pairs are as follows:
- NaOH (base) and Na+ (conjugate acid)
- H2O (acid) and OH– (conjugate base)
Amphoteric Substances
Water is amphoteric in the sense that it may behave as both an acid and a base.
A substance which can act as either an acid or a base is described as amphoteric.

The term is derived from the Greek amphoteros or amphoteroi, which imply “either or both of two” and, in essence, “either acid or alkaline.”
- H2O + H+ → H3O+
- H2O → H+ + OH−
The first reaction demonstrates that water is a base, while the second reaction demonstrates that water is an acid. As a result, it is amphoteric by definition. In water and aqueous solutions, these two processes occur spontaneously back and forth.
Amphoteric compounds can be recognized by attempting to remove or add hydrogen ions to the molecules. They are the “transitional phases” between acids and bases, and so appear frequently in acid-base chemistry studies, including neutralization reactions.
References
- Whitten, Kenneth; Davis, Raymond; Peck, Larry; Stanley, George (2013). Chemistry. Cengage Learning. p. 350. ISBN 978-1-133-61066-3.
- Masterton, William; Hurley, Cecile; Neth, Edward (2011). Chemistry: Principles and Reactions. Cengage Learning. p. 433. ISBN 978-1-133-38694-0.
- https://byjus.com/jee/bronsted-lowry-theory/
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Bronsted_Concept_of_Acids_and_Bases
- https://sciencenotes.org/bronsted-lowry-acid-and-base-theory/
- https://www.thoughtco.com/bronsted-lowry-theory-of-acids-and-bases-4127201
- https://opentextbc.ca/introductorychemistry/chapter/bronsted-lowry-acids-and-bases/
- https://www.studysmarter.us/explanations/chemistry/physical-chemistry/brnsted-lowry-acids-and-bases/
