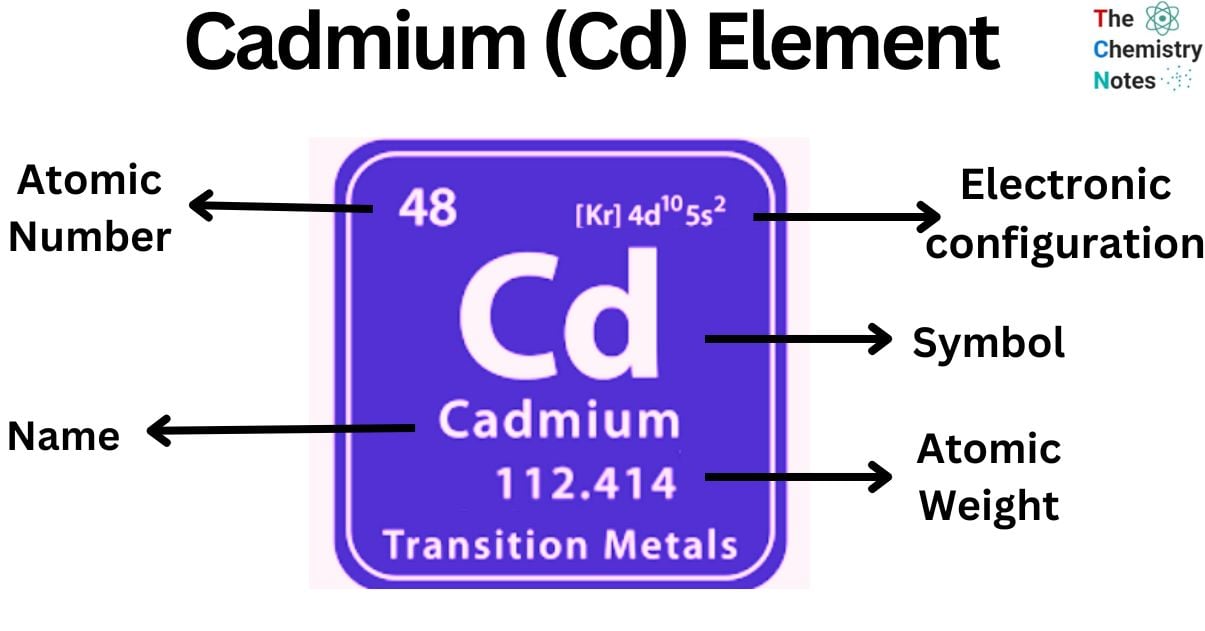
Cadmium is a chemical element with the atomic number 48 and is represented by the symbol ‘Cd’ in the periodic table. It belongs to the d-block of group 12 of the periodic table. It is soft and has a silvery white luster. It is not considered as a transition metal.
Cadmium is a rare metal with concentrations in the Earth’s crust ranging between 0.1 and 0.5 parts per million (ppm). It is a naturally occurring element.
Interesting Science Videos
History Of Cadmium
- Friedrich Strohmeyer, a German scientist, discovered cadmium in 1817 while investigating calamine samples.
- When heated, Strohmeyer discovered that some calamine samples burned yellow while others did not.
- He discovered that the calamine that changed color when heated included trace amounts of a new element after additional investigation.
- Name for the element is derived from the Greek word “calamine” (cadmium-bearing mixture of minerals) that was named after the Greek mythological character Cadmus.
Occurrence Of Cadmium
- Cadmium is a naturally occurring element in trace amounts. Its concentrations in the Earth’s crust range between 0.1 and 0.5 parts per million (ppm). It occurs naturally in mineral forms and is extracted commercially from cadmium ore known as greenockite (CdS).
- Greenockite is frequently discovered in conjunction with zinc ore. Cadmium is accessible commercially as an oxide, chloride, or sulfide.
- Cadmium is primarily produced as a byproduct of the mining, smelting, and refining of sulfidic ores of zinc.
- Cadmium can also be obtained through secondary sources. For instance, the dust produced by the recycling of iron and steel contains cadmium. It accounts for approximately 10% of total production.
Isotopes Of Cadmium
Cadmium consists of eight naturally occurring stable isotopes: 106Cd, 108Cd, 110Cd, 111Cd, 112Cd, 113Cd, 114Cd, and 116Cd.
Natural isotopes of cadmium
| Isotopes | Natural abundance (atom %) |
|---|---|
| 106Cd | 1.25 (6) |
| 108Cd | 0.89 (3) |
| 110Cd | 12.49 (18) |
| 111Cd | 12.80 (12) |
| 112Cd | 24.13 (21) |
| 113Cd | 12.22 (12) |
| 114Cd | 28.73 (42) |
| 116Cd | 7.49 (18) |
Elemental Properties Of Cadmium
| Electronic Configuration | [Kr] 4d10 5s2 |
| Atomic Number | 48 |
| Atomic Weight | 112.4 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 12, 5, d-block |
| Density | 8.65 g.cm -3 at 20 °C |
| Ionic radius | 0.097 nm (+2) |
| Van der Waals radius | 0.154 nm |
| Electron shells | 2, 8, 18, 18, 2 |
| Electrons | 48 |
| Protons | 48 |
| Neutrons in most abundant isotope | 68 |
Physical Properties of Cadmium
- Cadmium has an atomic number of 48 and is a silvery-white metal. It has a melting point of 321°C (610 °F) and a boiling point of 767°C (1413 °F).
- Cadmium has a solid phase density of 8.65 gm/cm3 and a liquid or molten phase density of 7.996 gm/cm3.
- It is an extremely malleable metal, which allows it to be easily hit into sheets without cleavage.
- It is also a ductile metal, which makes it possible to draw thin wires without breaking them. It is only second to gold in terms of ductility.
- Cadmium serves as a good electrical conductor. Its electrical conductivity is the greatest of all metals, even greater than that of copper. Because electrons in iron are free to move around they are able to carry electrical charge from one end to other.
- It is an extremely good thermal conductor, which is the highest among all other metals. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- Cadmium is soft metal that can be cut with a knife.
- It is highly corrosion resistant.
| Color/physical appearance | Silvery-White with bluish tinge |
| Melting point/freezing point | 321.069°C, 609.924°F, 594.219 K |
| Boiling point | 767°C, 1413°F, 1040 K |
| Density | 8.65 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.69 (Pauling Scale) |
Chemical Properties of Cadmium
- Bulk cadmium is insoluble in water and is not flammable, but powdered cadmium can ignite.
- Cadmium is commonly found in an oxidation state of +2. Certain compounds have also been discovered to exist in the +1 state as well.
- Cadmium does not react with water, however it does react with the majority of acids.
- Cadmium oxide is formed when cadmium interacts slowly with oxygen in wet air at ambient temperature.
Chemical Reaction of Cadmium
- The Reaction Of Cadmium With Air:
When cadmium metal ignites in the air, which results in cadmium (II) oxide. It depends on the manufacturing process that was carried out; different hues emerged from this element.
2 Cd (s) + O2 (g) → 2 CdO (s)
- The Reaction Of Cadmium With Water:
Cadmium does not react with water under normal conditions.
- The Reactions Of Cadmium With Halogens:
When cadmium metal reacts with fluorine (F2), the cadmium (II) difluoride CdF2 is formed.
Cd (s) + F2 (g) → CdF2 (s) [white]
When cadmium metal reacts with bromine (Br2), the cadmium (II) dibromide CdBr2 is formed.
Cd (s) + Br2 (g) → CdBr2 (s) [pale yellow]
When cadmium metal reacts with iodine (I2), the cadmium (II) diiodide CdI2 is formed.
Cd (s) + I2 (g) → CdI2 (s) [white]
Cadmium metal reacts with an aqueous solution of chlorine (Cl2).
Cd (s) + Cl2 (aq) → Cd2+ (aq) + 2 Cl− (aq)
- The Reaction Of Cadmium With Acids:
When cadmium metal (Cd) reacts with hydrobromic (Hbr) acid, it forms cadmium bromide (CdBr2) and hydrogen gas (H2).
Cd(s) + 2 HBr (aq) → CdBr2 (s) + H2 (g)
When cadmium metal (Cd) reacts with hydrochloric (Hcl) acid, it forms cadmium dichloride ( CdCl2 ) and hydrogen gas (H2).
Cd (s) + 2 HCl (aq) → CdCl2 (s) + H2 (g)
When cadmium metal is dissolved in dilute sulfuric acid, it produces solutions composed of the aquatic Cadmium (II) ion in addition to hydrogen gas in the form of H2. In actual experiments, the Cadmium (II) ion is found to be in the form of the complex ion [Cd(OH2)6]2+.
Cd (s) + H2SO4 (aq) → Cd2+ (aq) + SO42- (aq) + H2 (g)
The reactions of cadmium (Cd) metal with oxidizing acids like nitric acid (HNO3) are complicated and sensitive to the conditions present during the period during which the reaction occurs.
Uses Of Cadmium
There are wide applications for cadmium thanks to the extraordinary properties it possesses. Some of the applications of cadmium in modern society are discussed here:
Used In Batteries
The majority of the cadmium that is generated in modern times is utilized in the manufacturing of rechargeable batteries that are comprised of nickel-cadmium cells. Because cadmium is poisonous, these batteries have been phased out in favor of nickel-metal hydride and lithium-ion batteries in today’s technology.
Used In Alloys
The thermal and electrical conductivity of copper-based alloys can be improved by adding a small quantity of cadmium. The presence of cadmium reduces electrical arcing and enhances resistance to electrical erosion and material transfer. Because of its low coefficient of friction and fatigue endurance, cadmium is employed in a wide variety of bearing and solder alloys.
Used In Solar
Photovoltaic or solar cell applications utilize cadmium compounds, including cadmium telluride (CdTe) and cadmium sulfide (CdS). Camera exposure meters rely on cadmium sulfide photoconductive cells. To measure light, cameras use exposure meters using cadmium sulfide photoconductive cells. As a highly sensitive photoreceptor, cadmium sulfide has found application in photocopiers’ electrophotographic systems.
Used In Nuclear Industry
To regulate neutron flux during nuclear fission, cadmium is utilized as a neutron deactivator in the control rods of nuclear reactors. Cadmium absorbs neutrons in the core of a nuclear reactor, stopping them from causing new fission events and regulating the amount of reactivity. Silver-indium-cadmium alloys are employed as control rods in some pressurized water nuclear reactors, while cadmium sheet is used for radiation shielding due to its high neutron absorption qualities.
Used In Dye And Pigments
Paint pigments often contain cadmium salts, and the most commonly used yellow pigment is CdS. Cadmium red is a red pigment made of cadmium selenide. It is used for painting. They mix them with oils and binders or combine them with watercolors, gouaches, acrylics, and other paints and pigments.
Health Effects Of Cadmium
Some foods high in cadmium include dried seaweed, liver, mushrooms, shellfish, mussels, cocoa powder, and seafood. However, this absorption of cadmium by humans occurs only in trace amounts when these foods are consumed.
People who engage in the metal refining sector, along with those who reside nearby toxic waste dumps or facilities that emit cadmium into the environment, may also be exposed to the toxicity of the metal. Being exposed to cadmium can seriously harm a person’s lungs. This could result in mortality.
The effects of cadmium when consumed excessively on the human body are included here:
- Stomach discomfort, severe vomiting, and diarrhea
- Fractured bones
- Failure of the reproductive system, and potentially even infertility
- Central nervous systems injury
- Immune system dysfunction
- Mental illnesses
Cadmium is capable of being quantified in bodily fluids such as blood and urine, as well as in biological materials such as hair and nails. Research has demonstrated that urinary cadmium is a reliable indicator of the quantity of cadmium present within the human body. The concentration of cadmium present in an individual’s bloodstream serves as an indicator of their recent exposure to the aforementioned heavy metal. The urinary cadmium level serves as an indicator of both recent and historical exposure to cadmium.
Environmental Effects Of Cadmium
Due to the accelerated growth of modern industries and technologies, cadmium has been released into the environment.
- It is assimilated in significant quantities from sources such as water, food, and air pollutants.
- The presence of elevated levels of Cd has been observed in various marine organisms including crustaceans, bivalve mollusks, oysters, cephalopods, and crabs.
- Plant-derived foods typically exhibit elevated levels of Cd compared to animal-derived foods such as meat, eggs, milk, and dairy products, contingent upon the degree of soil pollution.
- Rice, wheat, green leafy vegetables, potatoes, carrots, and celery have been identified as potential sources of elevated levels of the aforementioned metal in plant-based food items.
- In aquatic habitats, cadmium may build up among mussels, oysters, shrimp, lobsters, and fish. The vulnerability to cadmium can differ substantially among aquatic animals. Salt-water species are more immune to cadmium poisoning than freshwater ones.
- Earthworms and many other essential soil species are susceptible to cadmium poisoning. They can die at low cadmium concentrations too, and this also has implications for the soil ecology. When cadmium concentrations in soils are high, they can alter the processes of microorganisms in the soil and imperil the entire soil ecosystem.
- Animals that consume cadmium occasionally through food or water get hypertension, liver sickness, and brain or nerve damage.
Video on Cadmium
References
- https://www.britannica.com/science/cadmium
- https://www.rsc.org/periodic-table/element/48/cadmium
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- https://www.lenntech.com/periodic/elements/cd.htm
- https://pubchem.ncbi.nlm.nih.gov/compound/Cadmium
- Helmenstine, Anne Marie, Ph.D. “Cadmium Facts.” ThoughtCo, Feb. 16, 2021, thoughtco.com/cadmium-element-facts-606511.
- https://www.cancer.gov/about-cancer/causes prevention/risk/substances/cadmium
- Giuseppe Genchi, Maria Stefania Sinicropi, Graziantonio Lauria, Alessia Carocci, and Alessia Catalano Effects of Cadmium Toxicity. doi: 10.3390/ijerph17113782 PMID: 32466586
- Wang Z, Sun Y, Yao W, Ba Q and Wang H (2021) Effects of Cadmium Exposure on the Immune System and Immunoregulation. Front. Immunol. 12:695484. doi: 10.3389/fimmu.2021.695484

