Californium is a synthetic chemical element with an atomic number of 98 and is represented by the symbol ‘Cf’ in the periodic table. It is silvery in appearance and belongs to the f-block of period 7 of the periodic table. Californium was identified as the sixth synthetic trans-uranium element in the actinide series. Similar to other elements in the actinide class, californium exhibits significant radioactivity. It is an explosive element found in almost all current nuclear weapons. American chemists Glenn T. Seaborg, Joseph W. Kennedy, and Arthur C. Wahl discovered curium in 1944. They created it by helium-ion bombardment of curium-242 in a 152-cm (60-inch) cyclotron followed by the separation from other elements by chromatography in Berkeley, California.
Interesting Science Videos
History of Californium
- Californium was synthesized by Stanley Thompson, Jr., Albert Ghiorso, Kenneth Street, and Glenn Seaborg.
- Thompson, Ghiorso, Street, and Seaborg led a research team at Berkeley, California, in 1950, that targeted curium-242 with helium ions to produce californium-245, which had a half-life of 44 minutes and contained a free neutron.
- Burris Cunningham and Stanley Thompson separated Californium in considerable quantities for the very first time in 1958 at the Materials Testing Reactor in Arco, Idaho, after five years of plutonium-239 neutron irradiation.
- The element was designated for the university and state of California, where it was discovered.
Occurrence of Californium
- Californium is a synthetic element that does not occur naturally on Earth. Californium was found to be produced in supernovae because its decay corresponds to the 60-day half-life of the 254-Cf element. However, later studies have failed to show any californium spectra.
- Californium is created in nuclear reactors by hitting plutonium with neutrons and in particle accelerators.
- Californium is thought to have occurred naturally on Earth in the natural nuclear fission reactor at Oklo; however, it no longer exists because all primordial isotopes of Californium have decayed.
- Curium-242 (half-life 162.8 days) was created by hitting plutonium-239 with alpha particles at Berkeley, California’s 60-inch cyclotron.
- Only around 5,000 atoms of californium-245 were synthesized throughout the experiment.
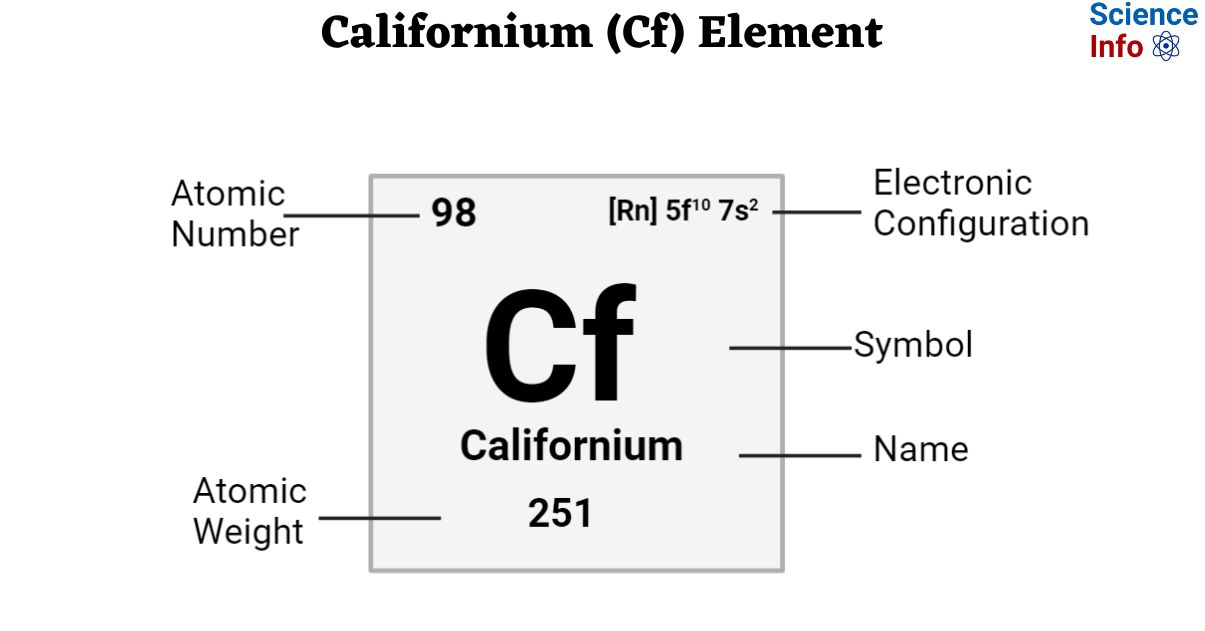
Elemental Properties of Californium
| Electronic Configuration | [Rn] 5f10 7s2 |
| Atomic Number | 98 |
| Atomic Weight | 251 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | 15.1 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 28, 8, 2 |
| Electrons | 98 |
| Protons | 98 |
| Neutrons | 153 (Varies with isotopes) |
Isotopic Information of Californium
- Californium does not have any naturally occurring isotopes.
- There are roughly 20 isotopes of californium with mass numbers ranging from 237 to 256, which half-lives are known.
- The heavier isotopes are created by intense neutron irradiation by the processes, whereas the isotope 249-Cf is a byproduct from the beta decay of 249-Bk.
- The occurrence of the isotopes 249-Cf, 250-Cf, 251-Cf, and 252-Cf allows for the isolation of californium in weighable amounts, allowing its microscopic properties to be studied.
- The longest-lived isotopes are 251-Cf (with a half-life of 898 years), 249-Cf (with a half-life of 351 years), and 250-Cf (with a half-life of 13.08 years).
Physical Properties of Californium
- Californium is a silvery-white, synthetic radioactive metal.
- It’s a somewhat soft, malleable metal that can be easily sliced with a razor blade.
- Californium has an atomic mass of 251.
- Californium has a melting point of 900°C. However, the boiling point is unavailable.
- It is the heaviest actinide and exhibits covalent characteristics comparable to Californium borate.
- Californium can be found in two different forms: face-centered cubic and double-hexagonal close-packed.
- Californium has ferromagnetic or ferrimagnetic properties below 51 K. It exhibits anti-ferromagnetism between 48 and 66 K. However exhibits paramagnetic properties over 160 K.
Chemical Properties of Californium
- Californium has the chemical characteristics similar to dysprosium, a lanthanide element.
- When exposed to room temperature, Californium tarnishes, and the rate of tarnishing increases with moisture; little chunks or foils of the metal begin to oxidize, although not violently.
- Californium-252 is an extremely powerful neutron emitter. One microgram emits 170 million neutrons every minute.
- It turns out that compounds containing californium exhibit oxidation states of +2, +3, and +4.
- Californium (III) is the sole ion that remains stable in aqueous solutions, with all attempts to decrease or oxidize it failing.
- Oxygen, nitrogen, chalcogens, and halogens are known to combine with californium to produce compounds.
- Californium compounds with an oxidation state of +3 share chemical characteristics with other actinide compounds with the same oxidation state.
- Californium compounds with an oxidation state of +2 are potent reducing agents.
- Californium compounds with an oxidation state of +4 are strong oxidizing agents.
- Due to the resistance of its compounds to reduction, a sample of the metal itself has yet to be produced. Acids, steam, and air are likely to do more damage than alkalis.
Production of Californium
- Californium is created using particle accelerators and nuclear reactors. Berkelium-249 is bombarded with neutrons, resulting in berkelium-250, which then beta decays to yield californium.
- When californium-250 is bombarded with neutrons, it creates californium-251 and californium-252.
- Curium isotopes are irradiated with neutrons in nuclear reactors, producing californium-252.
- Long-term irradiation of plutonium and americium to neutrons can yield a few mg of californium-252 and μg of californium-249.
Uses of Californium
- Californium has no applications in the chemical or industrial industries as of now.
- It is likely to find numerous new applications due to its high efficiency as a neutron source. It has already been used in neutron moisture gages and well logging (to determine water and oil bearing strata).
- Because of its neutron-emitting qualities, Cf is used in nuclear reactors as a neutron emitter and in a method referred to as neutron activation analysis in order to identify gold and silver ores.
- It’s also been utilized as a target material for transcalifornium production. Ununoctium, the heaviest element, was created when a californium target was hit with calcium ions.
- Californium is used in the aircraft industry and the military to identify faulty welds, hidden moisture, and corrosion in equipment.
- Cf-252 is utilized as a neutron source in therapies for brain and cervical cancer.
- Cf has a strong neutron yield making it an ideal kicking off neutron source for nuclear power plants.
Toxicity of Californium
- Californium-252 is an extremely powerful neutron emitter. It is known to be particularly radioactive. These are some of the potential health risks associated with radioactivity.
The primary issue associated with californium pertains more to its radioactivity rather than its chemical toxicity. Effectively managing the risk of californium exposure involves proper handling, containment, and the use of shielding. Stringent safety protocols and regulatory guidelines have been established to oversee the handling, utilization, and disposal of radioactive substances, californium included, with the aim of safeguarding both human health and the environment.
Health Effects of Californium
- Long-term exposure to the metal may be dangerous, yet no studies have shown that it causes cancer in humans.
- The most serious threat of radiation to life as we know it is damage to the gene pool, which is the genetic makeup of all living species. Radiation-induced genetic damage accumulates over time and across generations.
- Cancer can harm not only the individual but also the gene pool by transferring mutations.
Environmental Effects of Californium
- The advancement of nuclear technology has resulted on enormous as well as little emissions of radioactivity into the atmosphere, land, oceans, seas, and water table, which can be found in animal, vegetable, and inanimate materials around the planet.
- Radiation crosses species and concentrates in the food chain, harming other animals including humans.
Video Reference
References
- https://escholarship.org/content/qt10d7c1rs/qt10d7c1rs_noSplash_8e7de19f17ade71692905c12c0df1493.pdf?t=li5p55
- https://www.rsc.org/periodic-table/element/98/californium
- https://www.chemicool.com/elements/californium.html
- https://www.lenntech.com/periodic/elements/cf.htm
- https://www.livescience.com/40272-facts-about-californium.html
- https://www.americanelements.com/cf.html
- https://www.webelements.com/californium/
- https://www.chemistrylearner.com/californium.html
- https://chemicalengineeringworld.com/californium-element-properties-and-information/

