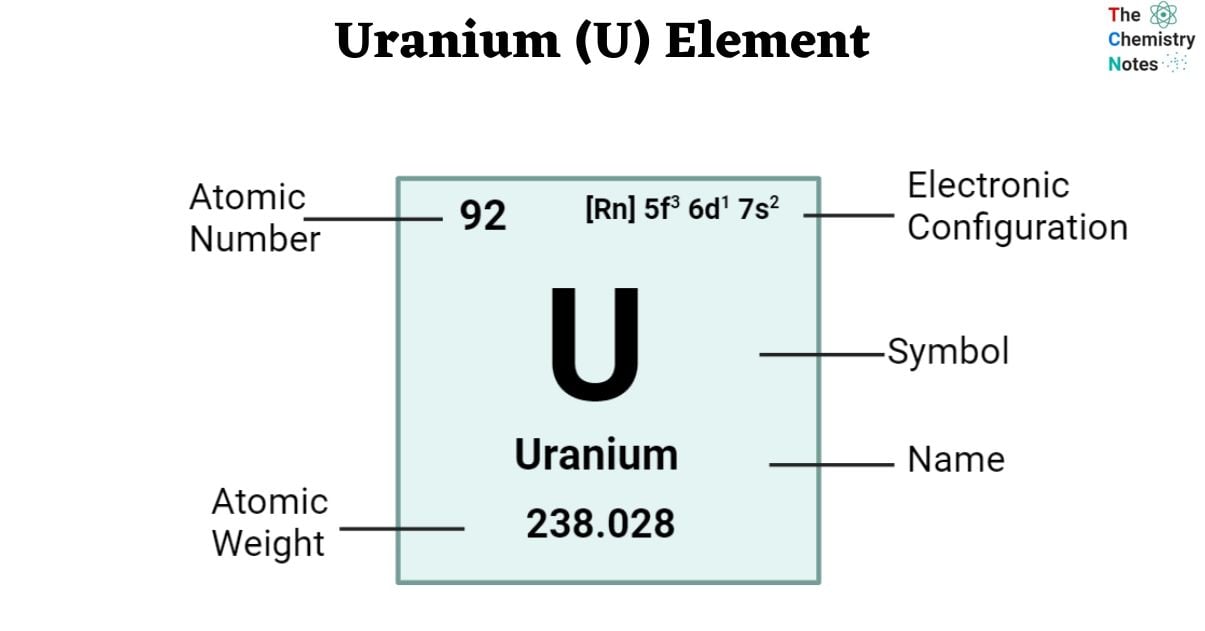
Uranium, a metallic chemical element found in the periodic table with atomic number 92 and denoted by the symbol U, displays a silvery-white appearance. Each uranium atom is characterized by 92 protons and 92 electrons, including 6 valence electrons. Notably, uranium boasts the highest atomic weight among naturally occurring elements. Uranium has been extensively used in the production of nuclear fuel for power plants, contributing significantly to the generation of electricity. However, its applications also extend to military purposes, where enriched uranium is utilized in the development of nuclear weapons.
Interesting Science Videos
History of Uranium
- Uranium’s discovery in 1789 is attributed to the German chemist Martin Klaproth, who identified an oxide of uranium while studying pitchblende samples from the Joachimsthal silver mines in the former Kingdom of Bohemia (present-day Czechia). Klaproth named the newly discovered element “uran” after the recently discovered planet Uranus.
- During the Middle Ages, pitchblende, a mineral containing uranium oxide (U3O8), was occasionally encountered in silver mines. In 1789, Martin Heinrich Klaproth conducted investigations on pitchblende, dissolving it in nitric acid and obtaining a yellow compound upon neutralization. Recognizing it as the oxide of a novel element, Klaproth attempted to produce the metal itself by heating the precipitate with charcoal, but his efforts were unsuccessful.
- The isolation of the first sample of uranium metal occurred in 1841 when Eugène Peligot in Paris successfully heated uranium tetrachloride with potassium.
- The revelation that uranium was radioactive occurred in 1896 when Henri Becquerel in Paris unintentionally left a sample of uranium on an unexposed photographic plate. The uranium caused the plate to become cloudy, leading Becquerel to conclude that uranium emitted invisible rays. This discovery marked the advent of radioactivity in scientific understanding.
Occurrence of Uranium
- Uranium is a relatively scarce element, constituting approximately two parts per million of the Earth’s crust.
- Its presence is notable in various minerals, with pitchblende (an impure form of U3O8), uraninite (UO2), carnotite (a potassium uranium vanadate), autunite (a calcium uranium phosphate), and torbernite (a copper uranium phosphate) being among the significant uranium-bearing minerals. These minerals, along with other recoverable uranium ores, serve as essential sources of nuclear fuels and possess a substantially higher energy potential compared to known recoverable deposits of fossil fuels.
- Uranium deposits manifest as a result of diverse geological processes, ranging from magmatic and fluid fractionation deep within the continental crust to evaporation at the Earth’s surface. The concentrations of uranium in different rock types exhibit a wide spectrum, varying from a fraction of a part per million in ultramafic rocks to 76 ppm in phosphorites.
Elemental Properties of Uranium
Physical Properties of Uranium
- Uranium is a heavy, lustrous, silvery-white metal, malleable, ductile, and slightly paramagnetic.
- Uranium, a metal in the actinide series, was formed over 6.6 billion years ago and is relatively rare in the solar system.
- Its slow radioactive decay, a major source of Earth’s heat, influences continental and convection drift.
- The high density of uranium is utilized in aircraft control surface counterweights and radiation shielding.
- Ranking among the heaviest naturally occurring elements, uranium is 18.7 times denser than water.
- Various isotopic forms of uranium exist, with natural uranium being a mixture of U-238 (99.3%) and U-235 (0.7%).
- In its pure state, uranium is silver, oxidizes readily in air, and is used to color glass that fluoresces greenish-yellow under black light.
- It exhibits three crystallographic modifications: alpha, beta, and gamma.
- The atomic mass of uranium is 238.03, and it has a melting point of 1132°C and a boiling point of 4131 °C.
- At 20°C, uranium has a density of 18950 in S.I. units.
- While malleable and ductile, uranium is hard enough to scratch glass and is a poor conductor of electricity.
- Uranium-235 is the only fissile isotope, with other naturally occurring isotopes being fissionable but not fissile.
- Handling uranium and its compounds requires caution due to their significant toxicity risks, both chemically and radiologically.
| Color/physical appearance | Silvery-white, metallic luster, but often tarnishes to a dull gray color. |
| Melting point/freezing point | 1405.3 K (1132.2 °C, 2070 °F) |
| Boiling point | 4404 K (4131 °C, 7468 °F) |
| Density | 19.05 g/cm³ |
| Flammability | Not Flammable |
| State of matter at room temperature | Uranium is a metal and is typically solid at room temperature |
Isotopic Properties of Uranium
- Natural Uranium has a 99.27 percent concentration of 238U, a 0.711 percent concentration of 235U, and very little 234U.
- Low Enriched Uranium – has a 235U content ranging from 0.711 percent to 20%. The majority of commercial reactor fuel is low enriched uranium (LEU) enriched to between 3% and 5% 235U. Uranium with a 235U content of 3 to 5% is commonly referred to as “reactor-grade uranium.”
- Highly Enriched Uranium – Uranium with a 235U concentration greater than 20%. Highly enriched uranium (HEU) is used in nuclear weapons, naval propulsion reactors, and some research reactors.
- Uranium that has been depleted has a 235U content of 0.711 percent or less. It is produced as a byproduct of the enrichment process.
Chemical Properties of Uranium
- When exposed to air, uranium creates a dark-colored coating on its surface.
- Almost all nonmetals and their compounds react with uranium metal.
- Uranium is a metal that is highly electropositive.
- Uranium dissolves readily in nitric and hydrochloric acids. Non-oxidizing acids eat away at uranium slowly.
- Cold water reacts with finely divided uranium.
Chemical Reaction of Uranium
- Reaction of uranium with acids: Acid dissolves uranium.
U(s) + 2 H+(aq) + 2 H2O(l) → UO22+(aq) + 3 H2(g)
U(VI) exists as yellow uranyl ions, UO22+, when [H+]> 10-2 M. At pH 3, the uranyle ion further hydrolyzes and dimerizes to [UO2(OH)]22+.
- Reaction of uranium with air: Uranium reacts with oxygen, O2, to form a thin layer of uranium oxide.
3 U(s) + 4 O2(g) → U3O8 (s) [white]
U(s) + 2 H+(aq) + 2 H2O (l) → UO22+(aq) + 3 H2(g)
- Reaction of uranium with carbonate: U(VI) is not precipitated by carbonate in the reaction of uranium with carbonate, but exists in complexes such as[ UO2(CO3)3]4−. Uranyle is precipitated as UO2(OH)2 by excess carbonate.
- Reaction of uranium with hydroxide ions: Alkalis have little effect on uranium. Hydroxide ions precipitate the uranyle ion. Uranyle is converted to di- and polyuranates by excess hydroxide ions.
UO22+(aq) + 2 OH-(aq) ⇆ UO2(OH)2(s) [yellow]
UO2(OH)2(s) + 2 Na+(aq) + 2 OH−(aq) → Na2U2O7(aq) + 3 H2O(l)
- Reaction of uranium with water: Oxygen-free water vapor and liquid water react with finely divided uranium
U(s) + 2 H2O(l) → UO2(s) + 2 H2(g)
2 U(s) + 3 H2(g) → 2 UH3(s)
- Reaction of uranium with hexacyanoferrate: Under acidic circumstances, potassium hexacyanoferrate precipitates the reaction of uranium with hexacyanoferrate UO22+ as ((UO2)2[Fe(CN)6] or UO2K2[Fe(CN)6] or a mixture of both.
2 UO22+(aq) + Fe(CN)64−(aq) → (UO2)2[Fe(CN)6](s) (red brown)
(UO2)2[Fe(CN)6](s) + 2 Na+(aq) + 6 OH−(aq) → Na2U2O7(aq) + Fe(CN)64−(aq) + 3 H2O(l)
Uses of Uranium
Uranium, a versatile element with a silvery-white metallic appearance, plays a crucial role in various applications across different industries. Here are some significant uses of uranium:
Energy Generation
- Widely employed in thermal and nuclear power plants globally.
- Conversion into Uranium dioxide allows the generation of thermal power, subsequently producing electrical energy through turbine-driven processes.
- Employed in light bulbs to prevent overheating, the production of dyes, various types of steel, and batteries as an electrical conductor.
- Uranium serves as a vital fuel for nuclear power plants, offering a highly efficient energy source.
- Enriched uranium, with higher concentrations of U-235, is crucial for nuclear power generation and weapons production.
Medicine
- Used in medical applications for treating cancers, AIDS, and certain types of anemia.
- Recognized as an antibiotic agent with contributions to medicine dating back to the 1940s, earning Dr. George Whipple a Nobel Prize.
Agriculture
- Utilized for sterilizing soil to prevent pest-related issues for farmers.
- Ensures the production of pesticides on a large scale after soil sterilization, albeit with careful consideration due to potential harmful effects.
Building Material
- Found in components of concrete, such as Alfred Nobel’s “Brick konkrete” from 1887, known for its high density and suitability as a load-bearing structure.
Nuclear Weapons
A key component in the creation of nuclear weapons due to its capacity to release significant energy rapidly.
Depleted Uranium
- A byproduct of uranium enrichment, depleted uranium, is used in military applications for its high density in shielding army tanks, parts of bullets, and missiles.
- Controversially, the use of depleted uranium in missiles raises concerns about the toxic vapor and dust formed upon impact.
Other Applications
- U-238 can be converted into fissionable plutonium in breeder reactors.
- Used for estimating the age of rocks (Uranium-238).
- Contributes to strengthening steel, inertial guidance devices, gyro compasses, aircraft control surfaces, missile reentry vehicle ballast, shielding, and x-ray targets.
Health Effects of Uranium
- Chemical Effects of Uranium on the Body: Both natural and depleted uranium exhibit identical chemical effects on the human body. Health impacts of uranium are primarily attributed to chemical reactions rather than radiation.
- Target Organs, Kidney Damage, and Respiratory Effects: Uranium predominantly affects the kidneys, with documented cases of kidney damage in humans and animals. Ingesting water-soluble uranium compounds can result in kidney effects at lower doses, while inhaled insoluble compounds may damage the respiratory tract.
- Limited Health Effects in Humans: Apart from kidney damage, no consistent health effects have been observed in humans exposed to uranium compounds or in soldiers with uranium fragments in their bodies. Long-term ingestion by rats has shown neurobehavioral changes and alterations in brain chemicals.
- Fertility and Skin Effects: Some studies suggest uranium’s potential to decrease fertility in rats and mice, though findings are not consistent across all studies. Very soluble uranium compounds on the skin can cause irritation and mild damage in animals.
- Carcinogenicity Classification: National Toxicology Program (NTP), International Agency for Research on Cancer (IARC), and EPA have not classified natural or depleted uranium regarding carcinogenicity.
- Children’s Exposure: Children, like adults, are exposed to uranium through air, food, and water. The specific health effects of uranium exposure on children remain uncertain, and it is unknown if they are more susceptible than adults.
- Pregnancy and Birth Defects: No strong human studies have shown birth defects due to uranium exposure. Some animal studies indicate potential toxicity in mothers leading to early deaths and birth defects in offspring, but results vary.
- Reducing Uranium Exposure: Recommendations include avoiding root vegetables from soils with high uranium levels and washing fruits and vegetables. If water is suspected to have elevated uranium levels, consider testing and potentially using bottled water. Caution is advised for those living near uncontrolled hazardous waste sites to prevent children from playing in contaminated dirt.
Environmental Effects of Uranium
- Uranium, a radioactive material, exhibits high reactivity, preventing its presence in the environment in its elemental form. Instead, it forms compounds through reactions with other elements and substances. These compounds dissolve in water, influencing their mobility and toxicity.
- The water-solubility of uranium compounds plays a crucial role in determining their environmental impact. It affects the distribution of uranium in the environment and influences its toxicity.
- While uranium itself is not inherently dangerous, certain decay products, notably radon, pose threats, especially in confined spaces such as basements.
- Uranium in the air exists as dust, which settles on surfaces through rainfall or settling. This dust can find its way into surface water, on plants, or on soils, eventually sinking into sediment or lower soil layers.
- Water containing low amounts of uranium is generally safe to drink. Uranium’s nature makes it unlikely to accumulate significantly in fish or vegetables, and any absorbed uranium is efficiently eliminated through urine and feces.
- Compounds in the soil can combine with uranium, with concentrations often higher in phosphate-rich soil. However, this usually falls within normal ranges for uncontaminated soil and doesn’t pose significant problems.
- Plants absorb uranium through their roots, and root vegetables like radishes may contain higher concentrations.
- Uranium is a naturally occurring radioactive material present in soil, rocks, water, and air. It oxidizes readily and is found in minerals rather than freely in the environment. Wind and water erosion redistribute uranium, and volcanic eruptions release it into the environment.
- Uranium can persist in the environment for years, with inactive mines, mills, and industries potentially continuing to release uranium. Understanding these dynamics is crucial for managing environmental impact.
- Uranium in the air exists as dust, and rain accelerates its settling onto water, plants, and land. This process influences the dispersion and concentration of uranium in various environmental compartments.
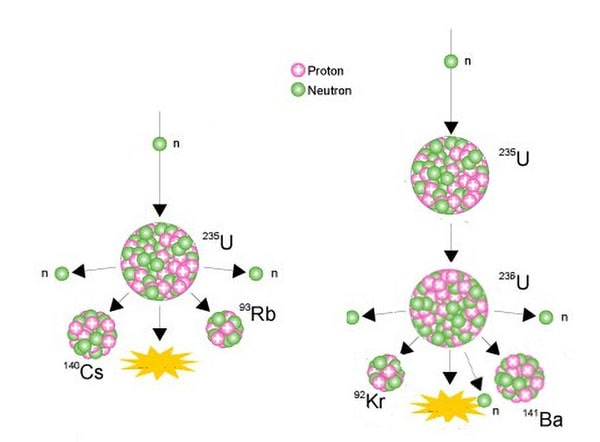
Nuclear Fission in Uranium
Uranium is also used to generate electricity via nuclear fission. When uranium nuclei split, they release a tremendous quantity of energy, which quickly translates to heat. When water fissions, the water heats and changes phases to steam, which can subsequently operate turbines and generate power. Nuclear scientists, on the other hand, must intensively process uranium ore in order to make useable nuclear fuel. The nuclear industry typically obtains uranium from yellowcake uranium ore (U3O8). Only 0.7% of the uranium atoms in yellowcake are uranium-235, the isotope required for reliable nuclear fission.
U-235 has 92 protons and 143 neutrons in its nucleus (92 + 143 = 235). When a U-235 atom’s nucleus grabs a traveling neutron, it splits in two (fissions) and releases energy in the form of heat, as well as two or three more neutrons. A fission ‘chain reaction’ can be established if enough of these expelled neutrons cause the nuclei of additional U-235 atoms to break, releasing further neutrons. When this occurs many millions of times, a significant quantity of heat is produced from a relatively tiny amount of uranium.In a nuclear reactor, this process of ‘burning’ uranium takes place. Heat is utilized to create steam, which is then used to generate electricity.
Video on Uranium
References
- https://www.chemistrylearner.com/uranium.html
- https://www.lenntech.com/periodic/elements/u.htm
- https://world-nuclear.org/information-library/nuclear-fuel-cycle/uranium-resources/the-cosmic-origins-of-uranium.aspx
- https://pilgaardelements.com/Uranium/Reactions.htm
- Helmenstine, Anne Marie, Ph.D. “Uranium Element Facts and Properties.” ThoughtCo, Feb. 16, 2021, thoughtco.com/uranium-facts-606616.
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- Tables of Physical & Chemical Constants, Kaye & Laby Online, 16th edition, 1995. Version 1.0 (2005), accessed December 2014.
- https://byjus.com/chemistry/uranium/
- J. S. Coursey, D. J. Schwab, J. J. Tsai, and R. A. Dragoset, Atomic Weights and Isotopic Compositions (version 4.1), 2015, National Institute of Standards and Technology, Gaithersburg, MD, accessed November 2016.
- T. L. Cottrell, The Strengths of Chemical Bonds, Butterworth, London, 1954.
- https://www.atsdr.cdc.gov/sites/toxzine/uranium_toxzine.html
- Keith S, Faroon O, Roney N, et al. Toxicological Profile for Uranium. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2013 Feb. 3, HEALTH EFFECTS. Available from: https://www.ncbi.nlm.nih.gov/books/NBK158798/
- https://world-nuclear.org/information-library/nuclear-fuel-cycle/introduction/what-is-uranium-how-does-it-work.aspx