A calorimeter is a device that measures heat, which is required for calorimetry. Calorimetry is the branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.
Interesting Science Videos
What is Calorimeter?
A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change.
The underlying principle is that the heat emitted by the combustion chamber causes a quantifiable increase in the temperature of the water. When compounds A and B react, the temperature change can be used to compute the enthalpy change per mole of substance A.
The formula is as follows:
q = Cv (Tf – Ti )
where:
q represents the amount of heat in joules.
Cv is the heat capacity of the calorimeter in joules per Kelvin (J/K).
Tf and Ti are the final and beginning temperatures, respectively.
In a basic calorimeter, the water is contained in a metal container that is placed above the combustion chamber, where a thermometer is used to track the water’s temperature change. There are, however, numerous different forms of more complicated calorimeters.
Types of Calorimeter
The following are the various types of calorimeters:
- Adiabatic Calorimeter
- Reaction Calorimeter
- Bomb Calorimeters (Constant Volume Calorimeter)
- Constant Pressure Calorimeter
- Differential Scanning Calorimeter
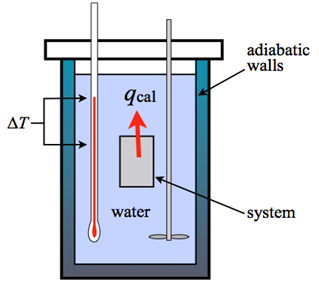
Adiabatic
An adiabatic calorimeter is one where heat is contained within the calorimeter, typically by enclosing it with an adiabatic shield that is kept at the calorimeter’s temperature.
In reality, the experiment involves a net heat exchange due to temperature gradients in the calorimeter and shield. In an adiabatic calorimeter, some heat is always lost to the container, but the estimate includes a correction factor to account for this heat loss. This type of calorimeter is used to study runaway reactions.
Reaction calorimeter
A reaction calorimeter is an instrument used in chemical and pharmaceutical development to measure the amount of energy emitted or absorbed by a chemical or physical process.
It typically consists of a stirred tank reactor with adjustable size, where the reaction mass’s temperature is precisely tracked and regulated along with all other crucial process parameters. The chemical reaction takes place inside a tightly sealed, insulated container in this kind of calorimeter. To determine the reaction heat, heat flow against time measurements are made. This is used to determine the maximum amount of heat emitted by a process meant to run at a constant temperature.
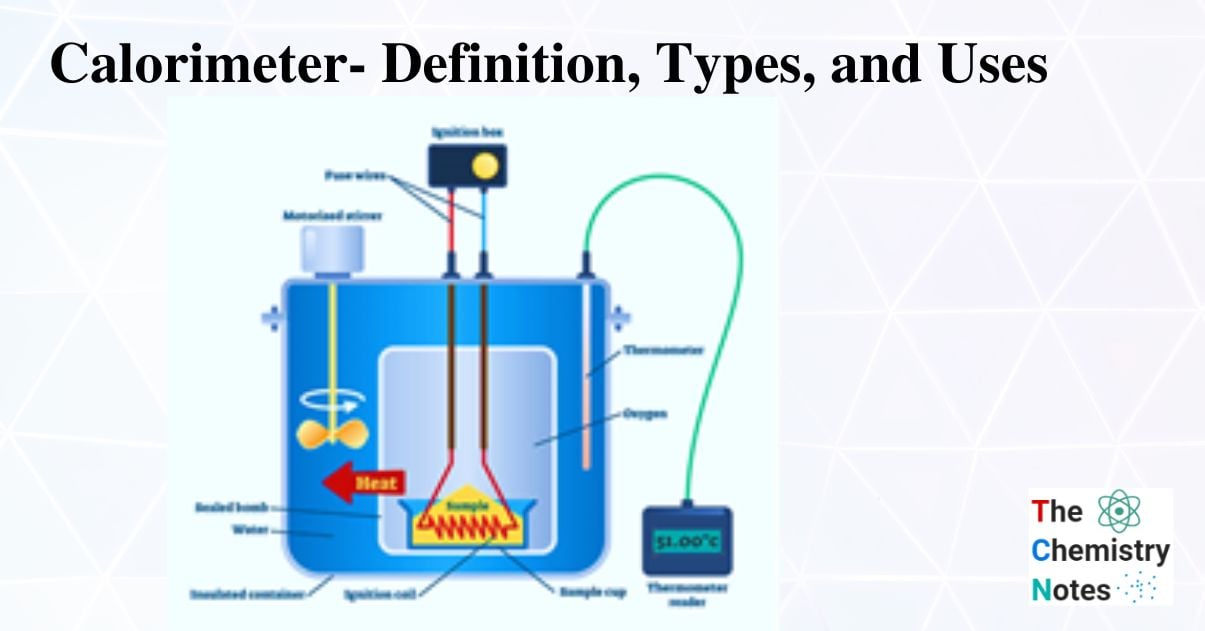
Bomb Calorimeters (Constant Volume Calorimeter)
A bomb calorimeter is a constant-volume calorimeter designed to resist the pressure created by the reaction as it heats the air within the container.
The heat of combustion is calculated using the temperature change of water. Bomb calorimeters are used to precisely calculate the energy shift that occurs during a reaction. The original Berthelot calorimeters was developed into the contemporary Bomb calorimeter. The combined bomb calorimeter of today is constructed of corrosion-resistant steel. An apparatus known as a bomb calorimeter is used to measure the heat of reaction at a constant volume and the measured heat, also known as the change of internal energy (E). (The name “bomb” derives from the observation that these reactions can be so intense that they mimic explosions that could harm other calorimeters.)
Working Principle
The combustion heat of samples that can burn with oxygen is measured using a specific kind of constant-volume calorimeter called a bomb calorimeter. Every bomb calorimeter needs four essential components.
A bomb calorimeter is a scientific tool used to quantify the heat produced during the burning of an excess of oxygen in a sample.
By recording the change in temperature during the reaction, one may calculate the heat change during a chemical reaction.
q = Cv (Tf –Ti)
where, q is the amount of heat according to the change in temperature measured in joules
Cv is the heat capacity of the calorimeters
Tf is the final temperature
Ti is the initial temperature
Use of Bomb Calorimeter
- It is used to measure the heat released during the combustion of a particular amount of biomass sample;
- To determine the Higher Heating Value (HHV) of that biomass fuel.
- By raising the temperature and the actual mass of the fuel, the total amount of heat produced is also determined.
- These are also important in professional and academic settings, to evaluate a change in a substance’s formula and its effects.
- Also Oxygen bomb calorimeters are helpful in food testing labs to determine the amount of heat (calories) in food.
Constant Pressure Calorimeter
A coffee cup calorimeter is its another name. It is a remarkably easy device to make; all you need is two Styrofoam cups, a stirrer, and a thermometer.
The two Styrofoam cups are placed inside of one another for insulation, and the inner cup undergoes a chemical reaction in a liquid medium, with the temperature change being recorded. Because the equipment is exposed to the atmosphere and records temperature change at constant pressure, it is known as a “constant pressure calorimeter.” The reaction mixture will probably expand or contract a little as the temperature changes, but volume change is essentially insignificant.
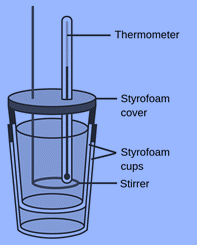
Hence, for a physical or chemical process, the change in enthalpy, H, is measured using constant-pressure calorimetry. This method uses a coffee cup, a cheap gadget made of two Styrofoam cups, to conduct a procedure in solution. The mass, specific heat, and temperature change of the solution can be used to compute the amount of heat transmitted throughout the process (q). q is equal to H during the process since the calorimeter is operating at constant (atmospheric) pressure.
Differential Scanning Calorimeter
Differential Scanning Calorimeter (DSC) is commercially accessible equipment that comes in two varieties: Heat Flux Type and Power Compensation. Heat Flux DSCs is a technique that varies the temperature of the sample unit, made up of a sample and reference material, according to a predetermined program, and thus measures the temperature difference as a function of temperature.
Power Compensation DSC is a method in which the temperature of the sample unit, is changed according to a predetermined program. The difference in thermal energy applied to the sample and reference material per unit of time is thus measured as a function of temperature to equalize their temperatures.
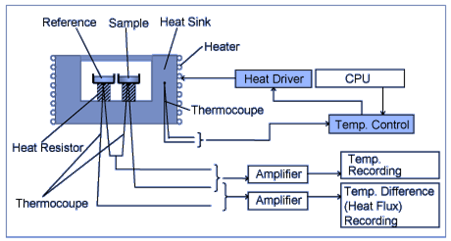
With the help of differential scanning calorimetry (DSC), heat flow into or out of a sample is measured as a function of temperature or time.
It is an extremely effective method for analyzing material characteristics such as glass transition temperature, melting, crystallization, and specific heat capacity.
Polymers, plastics, composites, laminates, adhesives, food, coatings, medicines, organic materials, rubber, petroleum, chemicals, explosives, biological samples. Thus variations in material composition, crystallinity, and oxidation are determined by the difference.
Uses
- Differential scanning calorimeters are used to determine the consequences of a change in a product’s formula.
- The heat produced by reactor sensors is measured using a reaction calorimeters.
- We can measure the change in enthalpy using both physical and chemical approaches using constant pressure calorimeters.
- Some of the fields of bomb calorimeters include waste product analysis, cement production, fuel analysis, and food and nutrition research.
- Oxygen Bomb Calorimeters, for instance, is used in the field of food testing to assist determine the food’s calorie content.
Read More: Thermodynamic Processes
References
- https://byjus.com/physics/calorimeter/
- https://www.thoughtco.com/definition-of-calorimeter-in-chemistry-604397
- https://www.hitachihightech.com/global/products/science/tech/ana/thermal/descriptions/dsc.html
- https://www.intertek.com/analysis/dsc/
- https://www.britannica.com/technology/calorimeter

