Carboxylic acids are the organic compounds containing the carboxyl group (-COOH) as a functional group.
The general formula is:
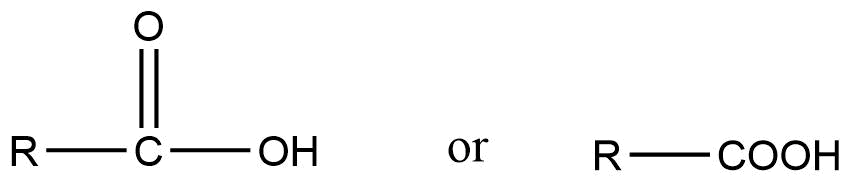
The name carboxyl is obtained from carbonyl (-CHO) and hydroxyl (-OH) as in the carboxyl group carbonyl and hydroxyl group are directly bonded to each other. These two groups combined gives the characteristic property of the (-COOH) carboxyl group.
Depending upon the number of -COOH groups present in carboxylic acid carboxylic acids are classified as monocarboxylic acid, dicarboxylic acid, and tricarboxylic acid.
- Monocarboxylic acids: Carboxylic acid containing one -COOH group. Eg: Acetic acid (CH3COOH)
- Dicarboxylic acid: Carboxylic acid contains two -COOH groups. Eg: Succinic acid (HOOCCH2CH2COOH)
- Tricarboxylic acid: Carboxyl acid containing two -COOH groups. Eg: Citric acid (CH2COOHCHOHCOOHCH2COOH)
Interesting Science Videos
Nomenclature of Carboxylic Acids
Common name system
Common names are usually obtained from the Greek word which indicates the original source of the acid. The common naming usually doesn’t follow any rule except all common names of acid end with -ic acid.
HCOOH Formic acid
CH3COOH Acetic acid
CH3CH2COOH Propionic acid
In substituted carboxylic acid common name, Greek letters i.e., α, β, γ are used to indicate the position of substituents. The carbon atom adjacent to the carboxyl acid is denoted by α, the next carbon on the chain is by β and the next one is γ.
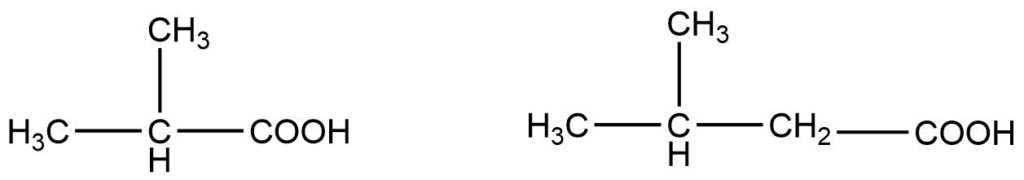
IUPAC system
In the IUPAC system, the naming of a carboxylic acid is done by replacing the ending -e of the corresponding alkene with -oic acid.
HCOOH – Methanoic acid
CH3COOH – Ethanoic acid
CH3CH2COOH – Propanoic acid
Methods of Preparation of Carboxylic Acids
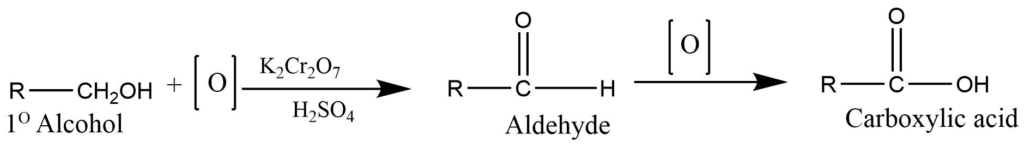
- Oxidation of primary alcohol or aldehyde: Primary alcohol or aldehyde on oxidation with potassium dichromate in presence of sulphuric acid produces carboxylic acid. Alcohol firstly gives aldehyde which on further oxidation gives carboxylic acid.
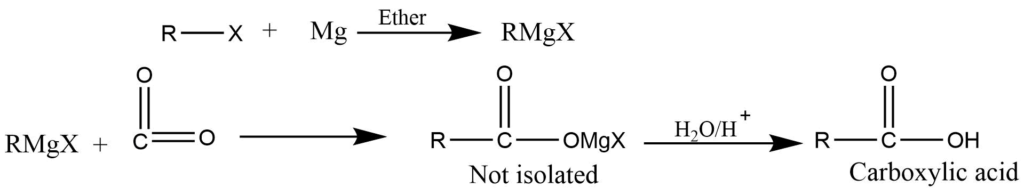
- Reaction of the Grignard reagent with CO2:Grignard reagent reacts with CO2 to give an addition product which on hydrolysis gives carboxylic acid.
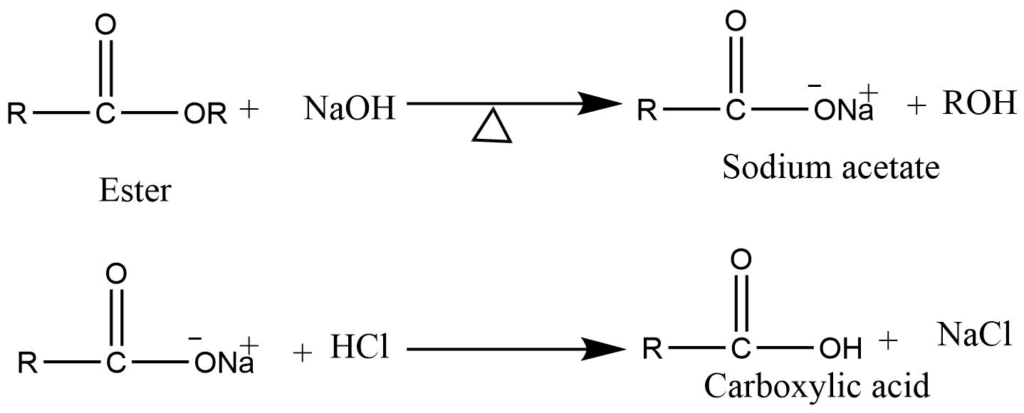
- Hydrolysis of ester: Reaction of the ester with NaOH produces sodium salt of carboxylic acid which on treatment with dilute HCl produces corresponding carboxylic acid.
- Carboxylation of alkene (Koch reaction): Alkenes on heating with CO and steam in presence of H3PO4 under the pressure and temperatureat 400oC form carboxylic acid. This is the industrial process of making carboxylic acid.
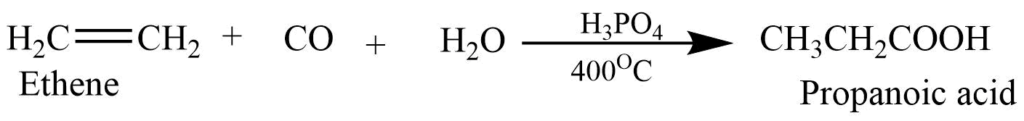
- Hydrolysis of nitriles: Nitriles are obtained by the reaction of an alkyl halide with hydrogen cyanide, on acid hydrolysis produces carboxylic acid.
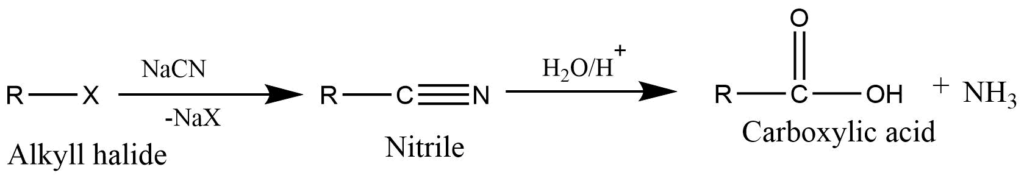
Physical properties of Carboxylic Acids
- Lower carboxylic acids (up to C10) are liquid disagreeable odors whereas higher members are wax-like and almost odorless.
- First four members are completely soluble in water but the water solubility decreases with an increase in the number of hydrocarbons in the chain.
- Boiling points of carboxylic acid are higher than those of alcohols having the same molecular weight.
| Name | Formula | Molecular weight | boiling point oC |
| Acetic acid | CH3COOH | 60 | 118 |
| 1-Propanol | CH3CH2OH | 60 | 97 |
- Boiling point of carboxylic acids increases with an increase in molecular weight.
- Melting point of carboxylic acids also increases with an increase in molecular weight.
| Name | Molecular weight | Melting point oC | Boiling point oC |
| Formic acid | 46 | 8.4 | 101 |
| Acetic acid | 60 | 16.6 | 118 |
Chemical properties of Carboxylic Acids
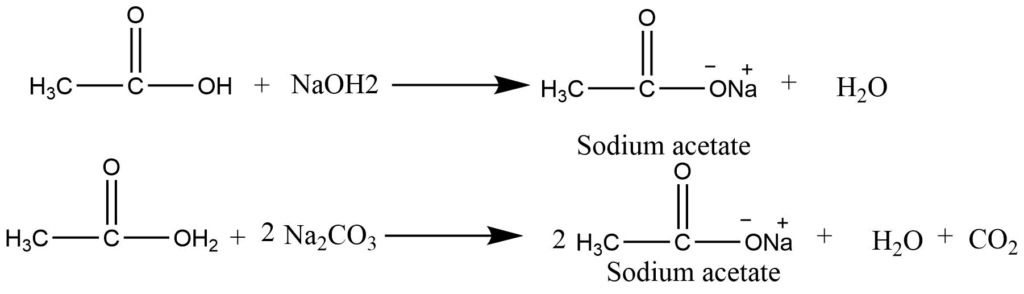
- Formation of salt: Carboxylic acids form salt on the reaction with hydroxides, carbonates, and bicarbonates.
- Formation of acid halide: Carboxylic acid on reaction with phosphorous halide and thionyl chloride forms acid halides.
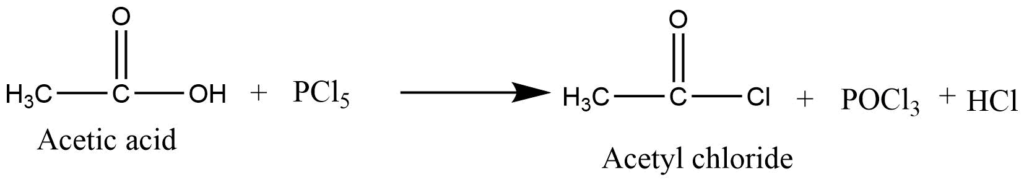
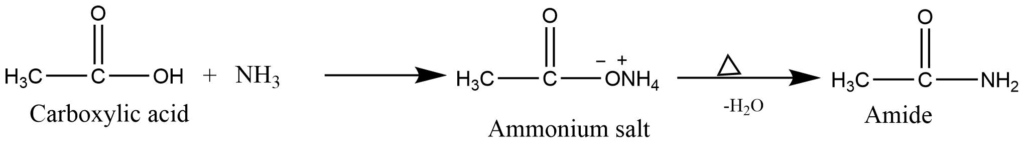
- Formation of amides: Reaction of a carboxylic acid with ammonia produces ammonium salt which on heating forms amide.
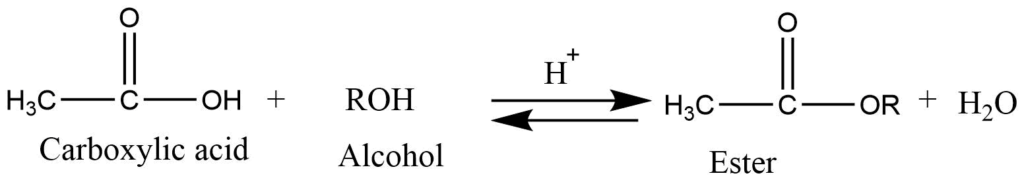
- Formation of ester (Esterification): In presence of strong acids like H2SO4, carboxylic acid reacts with alcohol to give ester. It is reversible reaction.
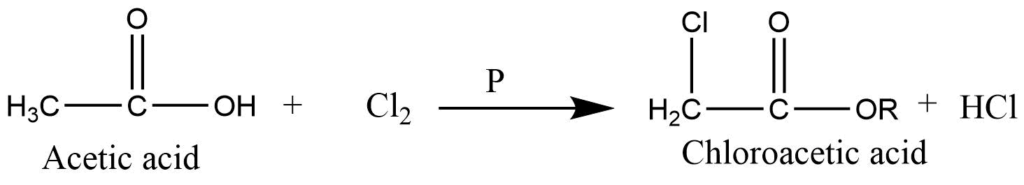
- Alpha halogenation (Hell-Volhard-Zelinsky reaction): Carboxylic acid with alpha hydrogen reacts with Cl2 or Br2 in presence of phosphorous to form the alpha substituted product. In this reaction, alpha hydrogen atoms are replaced by chlorine or bromine atoms.
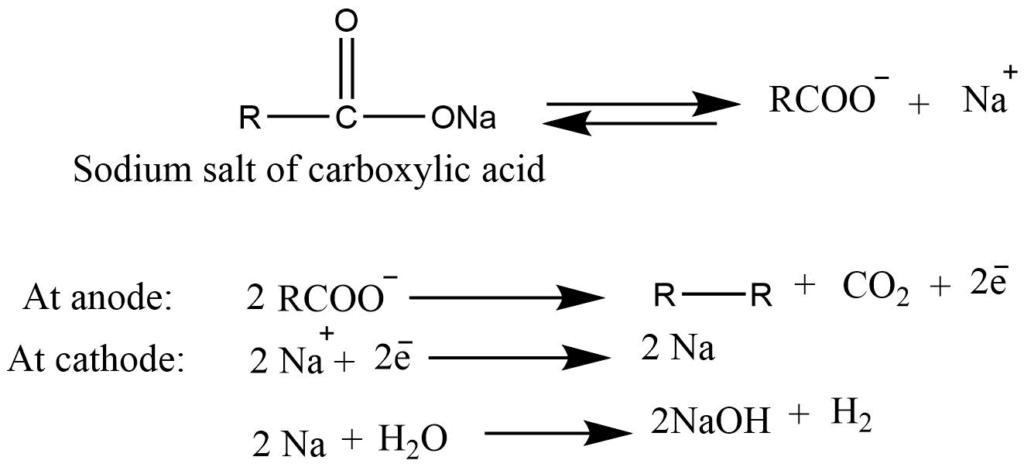
- Electrolysis (Kolbe’s electrolysis): Electrolysis of concentrated aqueous solution of the sodium salt of carboxylic acid gives alkene.
- Reaction of the silver salt of carboxylic acid (Hunsdiecker reaction): Silver salt of carboxylic acid on reaction with bromine or chlorine produces alkyl halide.
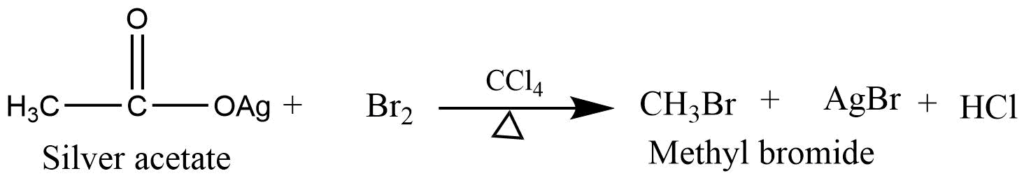
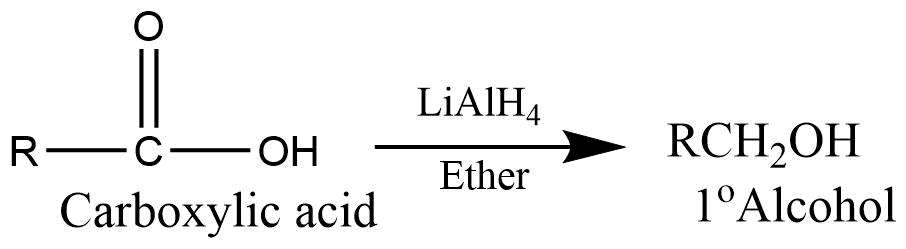
- Reduction: On reaction with lithium aluminium hydride carboxylic acid reduces to give primary alcohol.
- Formation of anhydride: In presence of phosphorous pentoxide carboxylic acid is dehydrated to give acid anhydride.
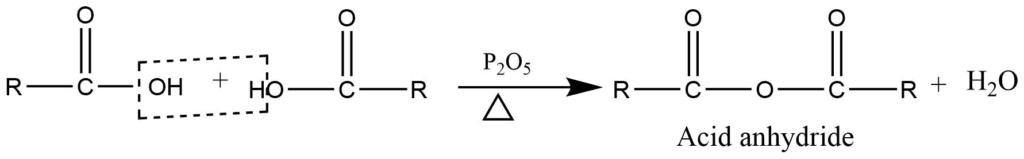
Uses of Carboxylic Acids
- Carboxylic acids are used in many organic synthesis reactions.
- Acetic acid is used as the solvent.
- They are used for making polymers, dyes, and perfumes.
- Higher fatty acids such as stearic acid are used for the production of soap.
- The sodium salt of carboxylic acids is used as a preservative.
- Carboxylic acid (acetic acid) is used as a coagulant for the manufacture of rubber.
- Many pharmaceutical drugs, such as aspirin and phenacetin, contain carboxylic acids.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- https://byjus.com/chemistry/carboxylic-acid-properties/
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/crbacid1.htm
- https://www.intechopen.com/chapters/61152
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Carboxylic_Acids
- https://byjus.com/chemistry/hunsdiecker-reaction/
- https://www.masterorganicchemistry.com/2020/09/30/the-hell-volhard-zelinsky-reaction/
- https://byjus.com/chemistry/uses-carboxylic-acid/
- https://www.britannica.com/science/carboxylic-acid/Synthesis-of-carboxylic-acids
- https://www.vedantu.com/chemistry/uses-of-carboxylic-acid
- https://www.toppr.com/ask/content/concept/uses-of-carboxylic-acids-203013/
