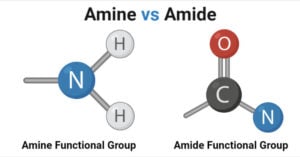
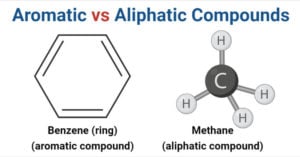
Aldehyde vs Ketone- Definition, 14 Key Differences, Examples
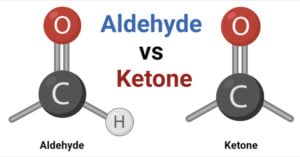
Aldehyde Definition An aldehyde is a type of organic compound containing the functional group with the structure –CHO, where the carbon double-bonded to oxygen is termed the carbonyl group. Aldehydes … Read more