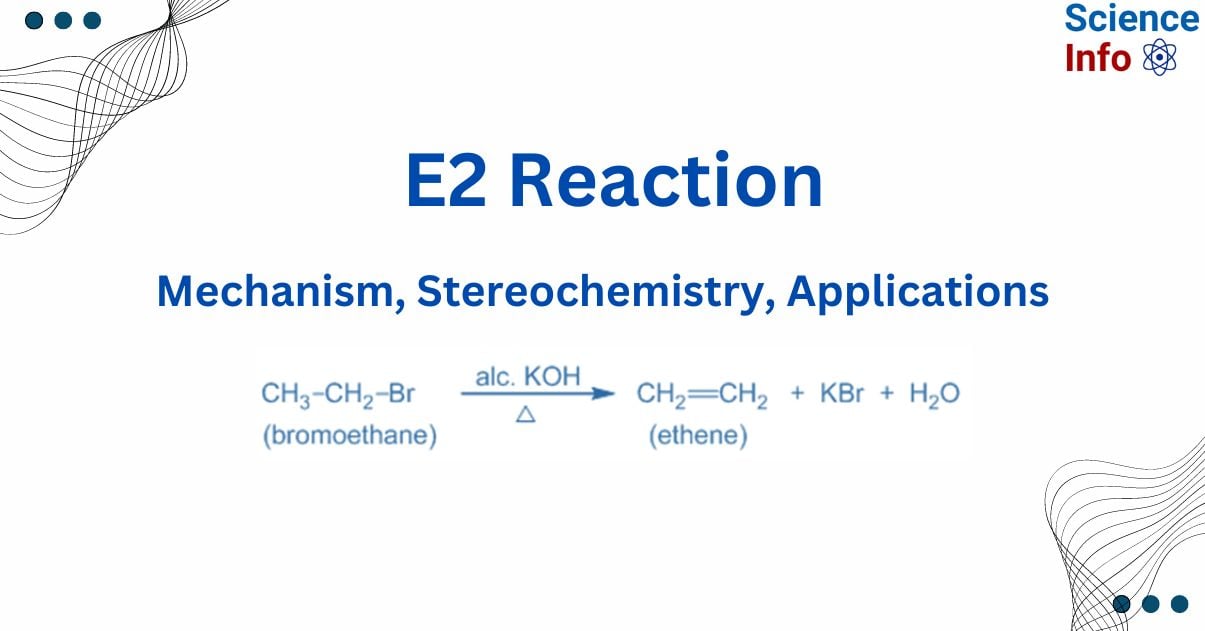
The E2 reaction process is a bimolecular elimination mechanism that removes beta-hydrogen and a leaving group from a molecule (substrate) simultaneously to generate a double bond. The removal often occurs in the presence of a strong base that removes the proton.
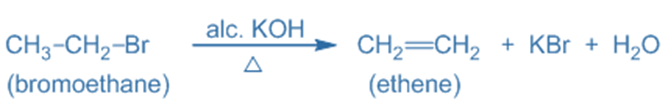
E2 reactions are categorized as “bimolecular” because they take place in a single step and involve the collision of two molecules: the substrate (often an alkyl halide) and the base. In the E2 reaction, a strong base (typically hydroxide ion, OH⁻) removes a proton from the carbon near the leaving group. As the base eliminates the proton, it also attacks the nearby carbon atom, aiding in the removal of the leaving group. This concerted reaction process causes the two carbon atoms to create a double bond (alkene). The proton is abstracted while the leaving group is released, ensuring that the number of atoms remains constant. For example: Dehydrohalogenation of alkyl halide
Interesting Science Videos
Kinetics of E2 reaction
A dehydrohalogenation reaction to produce alkene is a common example of an E2 reaction. The rate of reaction depends on the concentration of both alkyl halide and base. It follows second-order kinetics.
Rate α [Alkyl Halide] [Base]
R = k[RX] [:B]
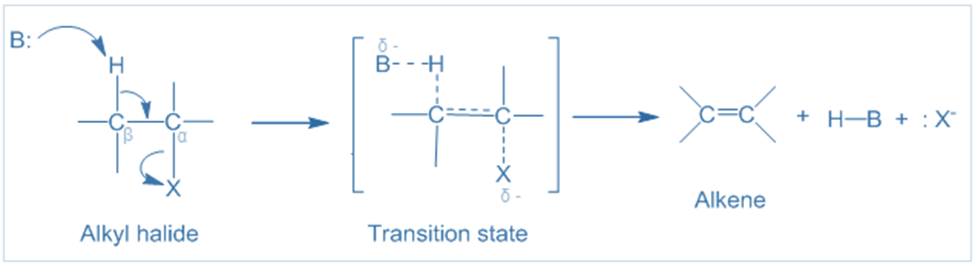
Mechanism of E2 reaction
The E2 mechanism is a single-step reaction process. During the reaction, the base attacks the β-carbon’s hydrogen atom and removes simultaneously carbon-carbon double bond forms, the -X group begins to leave, as seen in the transition state below. Following the transition state, the C-H and C-X bonds are entirely broken, and a carbon-carbon double bond is produced.
Regioselectivity and stereochemistry
In an E2 reaction, there may be several products. However, the more stable product formed according to Zaitsev’s rule is preferred. According to Zaitsev’s rule, the product with the most substituted carbon in the C=C pi bond will be the most thermodynamically stable.
The reactivity of the carbocation to the E2 reaction is as follows:
Tertiary > secondary > primary > methyl.
The bonds that bind the leaving group to the alpha-carbon and the hydrogen to the beta-carbon must be in the same plane. This orientation provides for adequate overlap of the two carbon orbitals, facilitating the creation of the pi bond. Furthermore, the hydrogen and the leaving group must be in opposite positions, i.e., in an anti-periplanar geometry.
This orientation allows the reaction’s transition state to be stable and low-energy. As a result, the hydrogen and leaving group must be in an anti-coplanar conformation. Because of the anti-coplanar structure, one stereoisomer (E isomer) will predominate over the other.
So, stereoselective reactions based on the E2 elimination mechanism create alkenes with the biggest groups on either end being trans to one another. Typically, this means that they selectively produce E-alkenes.
Factors affecting E2 elimination reaction
- Presence of R group
Increasing the number of R groups on the carbon with the leaving group leads to faster E2 reactions. The rise in E2 reaction rate with increased alkyl substitution can be explained in terms of transition state stability.
In the transition state, the double bond is only partially formed. A highly substituted alkene has a lower energy transition state, which reduces activation energy and speeds up the reaction.
- Nature of base
As the base’s strength increases, E2 reactions increase. E2 reactions frequently include a strong base. These compounds are typically unstabilized oxygen anions, such as hydroxides and alkoxides (CH3O- or CH3CH2O-). This includes very strong bases. Additionally, they include unstabilized carbon and nitrogen anions or semi-anions (e.g., amide, NH2, or alkyl lithiums like CH3Li or CH3CH2CH2CH2Li). Bases that have been stabilized in some way, such as resonance-stabilized carboxylates (like CH3CO2-) or enolates (like CH3COCH2-), are significantly weaker and are more likely to act as nucleophiles rather than bases.
- Solvent
Polar aprotic solvents, such as acetone, dimethyl sulfoxide (DMSO), or acetonitrile, are commonly used in E2 reactions because they do not considerably solvate the base, allowing it to stay strongly basic.
- Temperature
Unlike E1 reactions, which need higher temperatures, E2 reactions generally occur at moderate temperatures, about room temperature.
- Leaving group
E2 reactions occur more quickly when there is a stronger leaving group. The order of reaction rates is as follows: R-I > R-Br > R-Cl > R-F
Applications
E2 reactions have several practical applications in various chemical processes and industries. They are largely utilized in alkene synthesis, where chemists can make useful intermediates for medicines, polymers, and fine chemicals by removing a proton and releasing a leaving group. Another prominent application is dehydrohalogenation processes, which involve the removal of hydrogen halides from alkyl halides, which are required in the synthesis of many organic molecules. Furthermore, E2 reactions play an important role in polymer manufacturing, enabling the formation of polymers such as polypropylene and polyethylene.
Comparison between E1 and E2 reactions
Similarities
- Both are elimination reaction that involves the formation of double bonds.
Differences
- An E1 reaction is a chemical reaction in which a molecule is eliminated to produce double or multiple bonds, usually by the production of a carbocation intermediate. An E2 reaction, on the other hand, is a sort of organic chemical reaction in which a compound undergoes elimination to produce double or multiple bonds. In this reaction process, a proton and a leaving group are removed at the same time.
- The E2 reaction rate of reaction depends on the concentration of both substrate and base while the rate of reaction of E1 reaction depends on the concentration of substrate only.
- In the presence of a strong base, the rate of the bimolecular reaction will be faster so the E2 reaction will take preferently. In the presence of a weak base, the E1 elimination process is predominant.
- E1 reactions work better with tertiary substrates because the stability of the carbocation intermediate is important. E2 reactions are more favored with primary and secondary substrates because the accessibility of the proton to be abstracted is an important component.
- E1 reaction requires a good ionizing solvent while solvent polarity is not so important for the E2 reaction
- The E1 process begins with an ionization, which yields a flat carbocation. Ionization can occur without certain geometrical requirements. The E2 reaction occurs by a concerted mechanism that necessitates a coplanar arrangement of bonds between the atoms being removed. The transition state is often anti-coplanar, while it can be syn-coplanar in rigid systems.
- The E1 reaction includes a carbocation intermediate. This intermediate can rearrange, typically by shifting a hydride or alkyl group, to form a more stable carbocation. The E2 reaction occurs in one step, with no intermediates. The E2 reaction does not allow for any rearrangements.
- E2 reactions are usually faster than E1 reactions. In E2 reactions, a proton and a leaving group are eliminated simultaneously, resulting in a double bond. It does not require the formation of a stable carbocation intermediate, as E1 reactions do.
References
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Elimination_Reactions/E2_Reactions
- https://byjus.com/chemistry/elimination-reaction/
