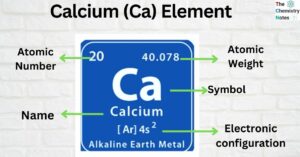
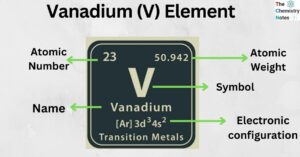
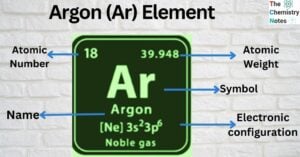
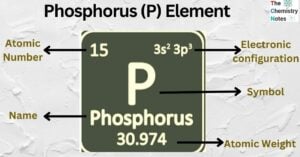
Calcium (Ca) Element: Properties, Uses, Amazing Facts
Calcium is a chemical element that belongs to Group 2 (IIa) of the periodic table and is represented as (Ca) in the periodic table. It is the fifth most plentiful element in the earth’s crust and the most abundant metallic … Read more