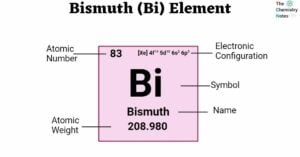
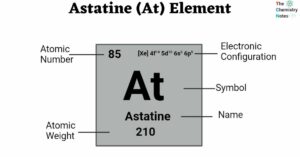
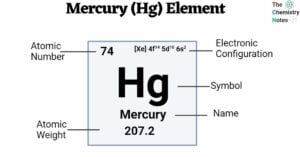
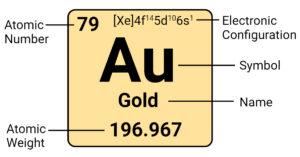
Actinium (Ac) Element: Properties, Reactions, Hazard
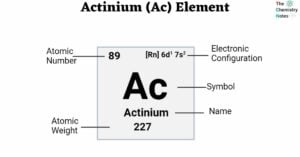
The atomic number of actinium is 89. It is found in f-block and Period 7 of the periodic chart. The symbol ‘Ac’ represents it. The name is derived from the ancient Greek word ‘aktis’, meaning beam or ray. Actinium is … Read more