Polonium is a chemical element with an atomic number of 84 and is represented by the symbol ‘Po’ in the periodic table. It is silvery in appearance and belongs to the p-block of group 16 of the periodic table. Polonium is the heaviest element within the chalcogen family. The remaining chalcogen elements consist of oxygen(O), sulfur (S), selenium (Se), and tellurium (Te). It is an extremely rare occurring element with no stable isotopes and is a highly radioactive metal.
Interesting Science Videos
History of Polonium
- Antoine-Henri Becquerel (1852–1908), a French scientist, identified a completely novel form of radiation that resembled light rays in 1898. It was discovered in the uranium ore pitchblende.
- Mendeleev predicted polonium by studying the periodic table long before Marie Curie discovered it. He predicted that the atomic weight of this element would be 212.
- Polonium was the first element discovered by Marie and Pierre Curie.
- They found polonium and later radium in 1898 in Paris while studying radioactivity in pitchblende (uranium oxide).
- Polonium is named after Marie Curie, who was born and raised in Poland.
Occurrence of Polonium
- Polonium is an extremely uncommon element due to the extremely short half-lives of all of its isotopes. It is found in trace amounts in uranium ores.
- Polonium is formed naturally when other radioactive elements degrade. However, it is so rare that all of the polonium required is currently produced in particle accelerators.
- It is created by impacting natural bismuth (Bi), 209Bi, with neutrons, resulting in 210Bi, which subsequently decays to 210Po through β decay.
- Every year, around 100 g of polonium is synthesized.
- Polonium has 29 isotopes with known half-lives, with mass numbers ranging from 190Po to 218Po. None of them are secure. The most stable isotope, having a half-life of 102 years, is 209Po.
Elemental Properties of Polonium
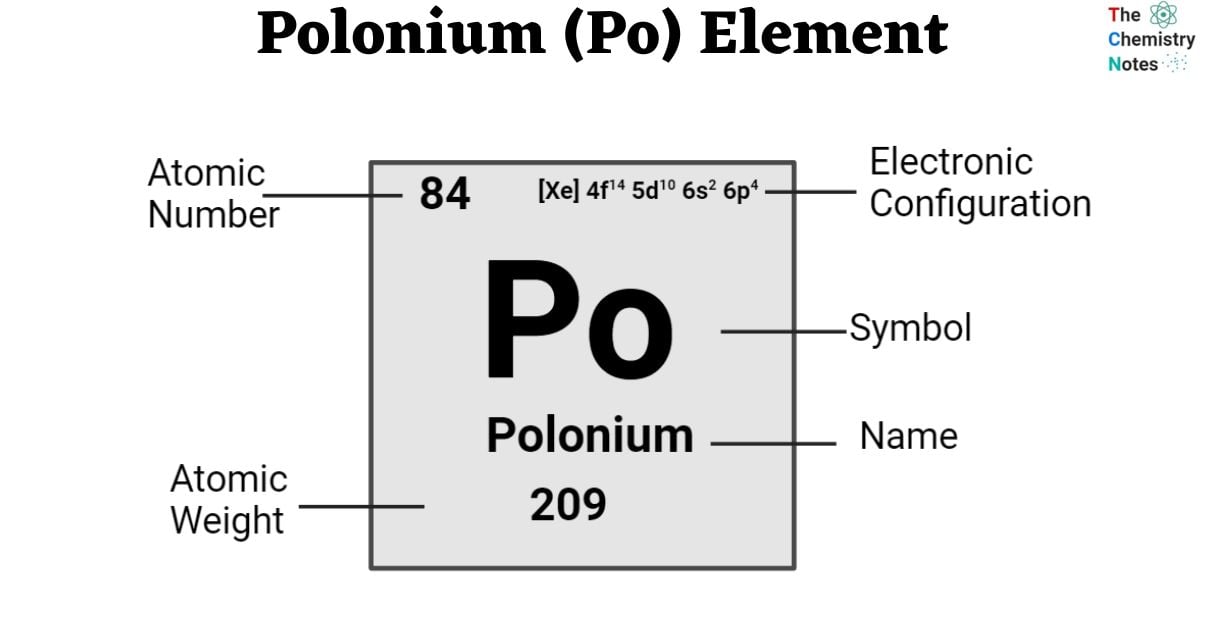
| Electronic Configuration | [Xe] 4f14 5d10 6s2 6p4 |
| Atomic Number | 84 |
| Atomic Weight | 209 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 16, 6, p-block |
| Density | 9.4 g/cm3 at 20 °C |
| Appearance | silvery |
| Van der Waals radius | 0.164 nm |
| Electron shells | 2, 8, 18, 32, 18, 6 |
| Electrons | 84 |
| Protons | 84 |
| Neutrons in the most abundant isotope | 125 |
Physical Properties of Polonium
- Polonium has an atomic number of 84 and is a silvery metal. It has a melting point of 254 °C (489 °F) and a boiling point of 962 °C (1764 °F).
- Polonium exists in two allotropic forms: simple cubic crystal structure and rhombohedral crystal structure.
- During radioactive decay, polonium emits a nucleus of helium (2 protons and 2 neutrons) in a process known as alpha decay.
- Po has a density of α-Po: 9.196 g/cm3 and β-Po: 9.398 g/cm3 at room temperature.
Chemical Properties of Polonium
- Polonium’s chemical characteristics are similar to those of the elements above it in the periodic table, particularly selenium and tellurium.
- Polonium is only partially soluble in alkalis.
- All polonium compounds are synthesized.
- Polonium dissolves quickly in dilute acids.
- Polonium is used to create an alloy with beryllium. The alpha particle that is emitted when polonium decays is caught by beryllium. Then, beryllium emits a neutron. Consequently, polonium and beryllium are mixed to produce a neutron source.
- Even though polonium solidifies at elevated temperatures, if heated in air to 55°C, it will evaporate.
- When polonium combines with hydrogen in the laboratory, PoH2 is produced.
Chemical Reaction of Polonium
- The Reaction of Polonium with Water
Polonium does not react with water.
- The Reaction of Polonium with Air
Polonium burns in the air to produce polonium (IV) oxide or PoO2.
[when heated Δ]
Po (s) + 2O2 (g) → PoO2 (s)- The Reaction of Polonium with Halogens
Polonium reacts under controlled conditions with the halogen chlorine, Cl2, to form the tetrahalides polonium (IV) chloride.
Po (s) + 2Cl2 (g) → PoCl4 (s) [yellow]Polonium reacts under controlled conditions with the halogen bromine, Br2, to form the tetrahalides polonium (IV) bromide.
Po (s) + 2Br2 (g) → PoBr4 (s) [red]Polonium reacts under controlled conditions with the halogen iodide, I2, to form the tetrahalides polonium (IV) iodide.
Po (s) + 2I2 (g) → PoI4 (s) [black]- The Reaction of Polonium with Acids
Polonium dissolves in a solution of concentrated hydrochloric acid, HCl, sulfuric acid, H2SO4, or concentrated nitric acid, HNO3, primarily to generate solutions containing Po (II).
Uses of Polonium
As a consequence of its exceptionally high radioactivity, polonium has limited commercial applications. Some of the most important applications of polonium are listed below:
- One application is to eliminate static electricity, which is an issue during the manufacturing of textiles, paper, and plastics. Positively charged alpha particles generated by polonium decay can reduce static electricity caused by an overabundance of electrons.
- Polonium has been employed in specialist equipment by scientists. A gram of polonium will reach 500°C as a result of the energy produced during radioactive decay. This heat has been employed as a power source in rockets.
- Polonium-210 can be mixed with a substance of a lower atomic weight element (typically Beryllium) to produce a metal alloy that can operate as a neutron emitter. One use for this characteristic is the creation of a neutron trigger for nuclear weapons.
Toxicity of Polonium
- Polonium is a radioactive material that generates very powerful alpha particles that can cause considerable harm to living cells.
- When consumed, breathed, or absorbed via the skin, it becomes a potent poison.
- When polonium enters the body, it attacks important organs such as the liver, kidneys, and bone marrow.
- It interferes with the regular functioning of these organs, resulting in serious health consequences and, in rare cases, death.
- Polonium’s alpha radiation destroys DNA in cells, producing mutations and altering biological functions.
- Leukemia, liver cirrhosis, stomach ulcers, bladder cancer, and cardiovascular disorders are among the illnesses that occur.
Environmental Effects of Polonium
- It is unknown what environmental and biological processes may tend to re-concentrate these hazardous compounds in live cells.
- Although polonium occurs naturally, it has become much more accessible to infiltrate water, food, living cells, and tissue since the mining boom that began immediately after WWII.
Video Reference
References
- https://www.rsc.org/periodic-table/element/84/polonium
- https://www.lenntech.com/periodic/elements/po.htm
- https://www.chemicool.com/elements/polonium.html
- https://www.livescience.com/39452-polonium.html
- https://www.americanelements.com/po.html
- https://www.thoughtco.com/polonium-facts-element-84-or-po-606577
- https://chemicalengineeringworld.com/polonium-element-properties-and-information/
- https://winter.group.shef.ac.uk/webelements/polonium/chemistry.html#:~:text=Polonium%20reacts%20under%20controlled%20conditions,and%20polonium(IV)%20iodide.
- https://lambdageeks.com/polonium-uses/

