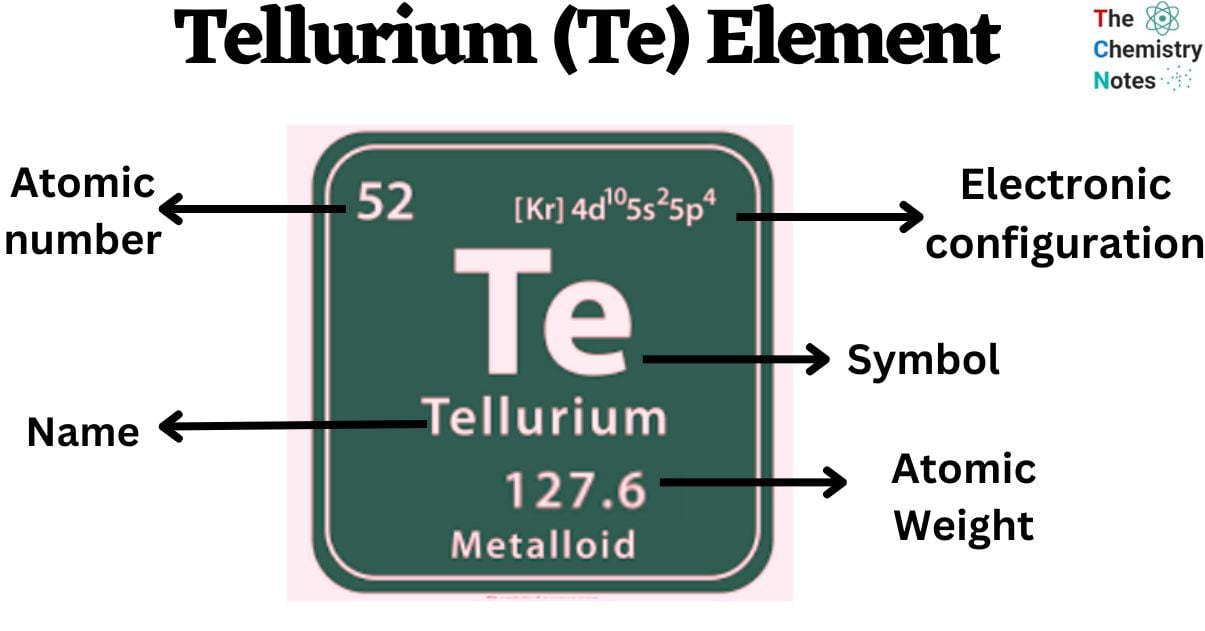
Tellurium is a chemical element with the atomic number 52 and is represented by the symbol ‘Te’ in the periodic table. It is classified as a metalloid and belongs to the p-block of group 16 of the periodic table. It is a rare silvery-white semimetal. It exists in two different allotropic forms: crystalline and amorphous.
Tellurium is one of the rarest elements. Its presence on the Earth’s crust is approximately 1 ug/L (1 part per billion), which is rarer than thulium and rubidium. It has eight naturally occurring isotopes, out of which six are stable.
Interesting Science Videos
History of Tellurium
- In the 18th century, tellurium was found in gold ore from the mines at Kleinschlatten, close to the modern Romanian city of Alba Iulia. It was isolated even before it was recognized as an element.
- Franz Joseph Müller von Reichenstein, an Austrian mineralogist, worked with an ore known as German gold in 1782. He extracted a substance from this ore that eluded his attempts at the examination, which he labeled metallum problematicum.
- In 1789, A Hungarian scientist named Pál Kitaibel also found the element in ore from Deutsch-Pilsen that had previously been thought to be argentiferous molybdenite, although he ultimately gave Müller the credit.
- Martin Heinrich Klaproth verified Müller’s observations and established the substance’s elemental origin in 1798.
- Tellurium gets it name from the Latin word “telus”,meaning Earth.
Occurrence of Tellurium
- Tellurium is one of the rarest elements. Its presence on the Earth’s crust is approximately 1 ug/L (1 part per billion), which is rarer than thulium and rubidium.
- Tellurium is occasionally found in its elemental form in nature, but it is more frequently found as the tellurides of gold and silver, including minerals such as calaverite (a form of gold telluride), sylvanite (silver-gold telluride), and tellurite (tellurium dioxide).
- The only naturally occurring chemical forms of gold are tellurium compounds. Contrary to gold, however, tellurium itself can also be discovered in combination with other substances, forming metallic salts.
- Tellurium, which is mainly produced today, is a byproduct of the purification of blister copper. 500 tons of copper ore are processed to produce 0.45 kilograms of tellurium. Impure elements accumulate as anode mud, which contains around 8% tellurium, during the electrolytic purification of copper.
- The United States, Japan, Peru, and Canada are among the key producers of tellurium.
Isotopes of Tellurium
There are 30 known isotopes of tellurium. It consists of eight naturally occurring stable isotopes: 120Te, 122Te, 123Te, 124Te, 125Te, 126Te, 128Te, and 130Te.
Naturally Occurring Isotopes of Tellurium
| Isotopes | Natural abundance (atom %) |
|---|---|
| 120Te | 0.09 (1) |
| 122Te | 2.55 (12) |
| 123Te | 0.89 (3) |
| 124Te | 4.74 (14) |
| 125Te | 7.07 (15) |
| 126Te | 18.84 (25) |
| 128Te | 31.74 (8) |
| 130Te | 34.08 (62) |
Elemental Properties of Tellurium
| Electronic Configuration | [Kr] 4d10 5s2 5p4 |
| Atomic Number | 52 |
| Atomic Weight | 127.60 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 16, 5, p-block |
| Density | 6.24 g.cm -3 at 20 °C |
| Ionic radius | 0.221 nm (-2) ; 0.089 nm (+4) |
| Van der Waals radius | 206 pm |
| Electron shells | 2, 8, 18, 18, 6 |
| Electrons | 52 |
| Protons | 52 |
| Neutrons in most abundant isotope | 76 |
Physical Properties of Tellurium
- Tellurium has an atomic number of 52 and is a silvery-white metalloid. It has a melting point of 449.51 °C (841.12 °F) and a boiling point of 988 °C (1810 °F).
- Tellurium has a solid phase density of 6.24 gm/cm3 and a liquid or molten phase density of 5.70 gm/cm3.
- It is poor heat conductor and semi-conductor of electricity.
- Tellurium is brittle and easy to pulverize.
| Color/physical appearance | Silvery-white |
| Melting point/freezing point | 722.66 K, 449.51 °C, 841.12 °F |
| Boiling point | 1261 K, 988 °C, 1810 °F |
| Density | 6.24 g cm-3 at 20° |
| Malleability | No |
| Ductility | No |
| Electronegativity | 2.1 (Pauling Scale) |

Chemical Properties of Tellurium
- Hydrochloric acid has no effect on it; however, either nitric acid or aqua regia (a solution of nitric acid and hydrochloric acid) will cause it to oxidize into tellurous acid (H2TeO3).
- It combines with most metals at high temperatures to generate tellurides and reacts with the halogens (fluorine, chlorine, bromine, and iodine) to form halides.
- Tellurium produces dioxide (TeO2) when it burns in air or oxygen with a blue-green blaze.
- Tellurium can be bonded to other elements to create tellurides, halides, oxo- compounds, and organotellurium compounds.
Chemical Reaction of Tellurium
- The Reaction of Tellurium With Air
Tellurium reacts with oxygen in the atmosphere to produce tellurium(IV) oxide, often known as TeO2, when it is burned.
Te (s) + 2 O2 (g) → TeO2 (s)
- The Reaction of Tellurium With Water
Under typical circumstances, there is no reaction between tellurium and water.
- The Reaction of Tellurium With Halogens
Tellurium, when combined with fluorine, F2, and then heated, produces the hexafluoride tellurium(VI) fluoro as a by-product.
Te8 (s) + 24 F2 (g) → 8 TeF6 (l) [orange]
Tellurium, when allowed to react with fluorine, F2, in the presence of nitrogen gas at a temperature of 0 degrees Celsius, produces the tetrafluoride tellurium (IV) fluoride.
Te8 (s) + 16 F2 (g) → 8 TeF4 (s)
Tellurium reacts under controlled conditions with chlorine, Cl2, to form tetrahalides of tellurium (IV) chloride.
Te8 (s) + 16 Cl2 (g) → 8 TeCl4 (s)
Tellurium reacts under controlled conditions with bromide, Br2, to form tetrahalides of tellurium (IV) bromide.
Te8 (s) + 16Br2 (g) → 8 TeBr4 (s) [red]
Tellurium reacts under controlled conditions with iodine, I2, to form tetrahalides of tellurium (IV) iodide.
Te8 (s) + 16 I2 (g) → 8 TeI4 (s)
- The Reaction of Tellurium With Acid
Tellurium does not interact with diluted non-oxidizing acids in any way.
Uses of Tellurium
- Tellurium is most commonly used in alloys of iron, stainless steel, copper, and lead, which are produced by the metalworking industry. The combination of steel and copper results in the formation of an alloy that can be machined more easily than other metals. Tellurium enhances the properties of lead, making it more durable and longer-lasting while also lowering the metal’s susceptibility to corrosion caused by sulfuric acid.
- Tellurium is added to copper to enhance machinability. Compared to pure copper, tellurium-copper alloys are also simpler to deal with. Additionally, there is no change in copper’s fundamental capacity to conduct an electric current. Lead also has tellurium added to it. Tellurium-lead alloys are stronger than pure lead in terms of vibration and fatigue resistance. Metal fatigue is the propensity for metal to degrade over time through repeated use.
- Tellurium can be found in a variety of photocathodes, including those that are utilized in solar-blind photomultiplier tubes and high-brightness photo-injectors that power contemporary particle accelerators. The CsTe photocathode, which is predominantly composed of Cs2Te, has a photoemission threshold of 3.5 eV and demonstrates a unique combination of high quantum efficiency (more than 10%) and great endurance in situations with low levels of vacuum pressure.
- Tellurium has the potential to be used in cadmium telluride (CdTe) solar panels. Using this material to make solar cells that make electricity has led to some of the best results. Since First Solar Inc. began large-scale commercial manufacture of CdTe solar panels in recent years, tellurium demand has skyrocketed. When some of the cadmium in CdTe is replaced by zinc, CdZnTe is created, which is employed in solid-state x-ray detectors.
- Tellurium is being used in more and more electrical equipment. For instance, it is applied to enhance picture quality in printers and photocopiers. Infrared detection devices also employ a tellurium, cadmium, and mercury combination. Heat is the infrared spectrum. Special glass can be used to make it visible. Some Earth-orbiting satellites measure the infrared radiation that plants emit in order to research forests, crops, and other plant life.
- In the rubber and textile sectors, tellurium is used in around 15% of total production. It is crucial, for instance, in the vulcanization of rubber. Soft rubber is transformed into a harder, more durable product by the process of vulcanization. The production of synthetic fibers also uses tellurium as a catalyst. A catalyst is a substance that is used to accelerate or inhibit a chemical reaction without changing chemically.
- High-purity tellurium is being used more frequently in the electronics industry for a variety of novel and developing applications. For instance, tellurium is utilized in rewritable CD, DVD, and Blu-ray discs along with the recently created phase-change memory chips.
- Thermoelectric cooling devices frequently use bismuth telluride. These gadgets have a wide range of uses in consumer goods and technology, with portable food coolers and, it’s hard to believe, even car seat chilling systems seeing growing use of tellurium recently.
Health Effects of Tellurium
- The majority of people, fortunately, do not come into contact with tellurium compounds very often. They are teratogenic and should only be handled by qualified chemists since ingestion of even a small amount creates awful-smelling breath and atrocious body odor.
- At 20 degrees Celsius, there is almost no evaporation; nonetheless, a potentially dangerous concentration of airborne particles can be achieved very quickly when the substance is disseminated. Inhalation results in several consequences: Drowsiness. a lack of saliva in the mouth, a metallic taste, a headache, and nausea.
- The aerosol form of this chemical is irritating to the respiratory tract as well as the eyes. The chemical may affect the liver in addition to the central nervous system. Constipation, vomiting, and pain in the abdomen are caused by ingestion.
- The lithium silicide produces an ignitable reaction with tellurium. Finely disseminated particles create explosive airborne mixtures.
Environmental Effects of Tellurium
The Earth’s crust and oceans contain very small amounts of naturally occurring tellurium, which can be poisonous in some forms. Tellurium is classified as a metalloid, a chalcogen group chemical element with no recognized biological function. However, both prokaryotic and eukaryotic species are adversely affected by its constituents, particularly the oxyanions. Recent research reveals that autoimmune, neurological, and oncological disorders are causally related to tellurium contamination of the environment.
Video on Tellurium
References
- https://lambdageeks.com/tellurium-chemical-properties/
- https://www.rsc.org/periodic-table/element/52/tellurium
- https://www.britannica.com/science/tellurium
- https://byjus.com/chemistry/tellurium/
- Greenwood, N. N. & Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
- Emeleus, H. J. (1990). A. G. Sykes (ed.). Advances in Inorganic Chemistry. Vol. 35. Academic Press. ISBN 0-12-023635-4.
- Holloway, John H.; Laycock, David (1983). “Preparations and Reactions of Inorganic Main-Group Oxide-Fluorides”. In Harry Julius Emeléus; A. G. Sharpe (eds.). Advances in inorganic chemistry and radiochemistry. Serial Publication Series. Vol. 27. Academic Press. p. 174. ISBN 0-12-023627-3.
- Vávrová S, Struhárňanská E, Turňa J, Stuchlík S. Tellurium: A Rare Element with Influence on Prokaryotic and Eukaryotic Biological Systems. Int J Mol Sci. 2021 May 31;22(11):5924. doi: 10.3390/ijms22115924. PMID: 34072929; PMCID: PMC8199023.

