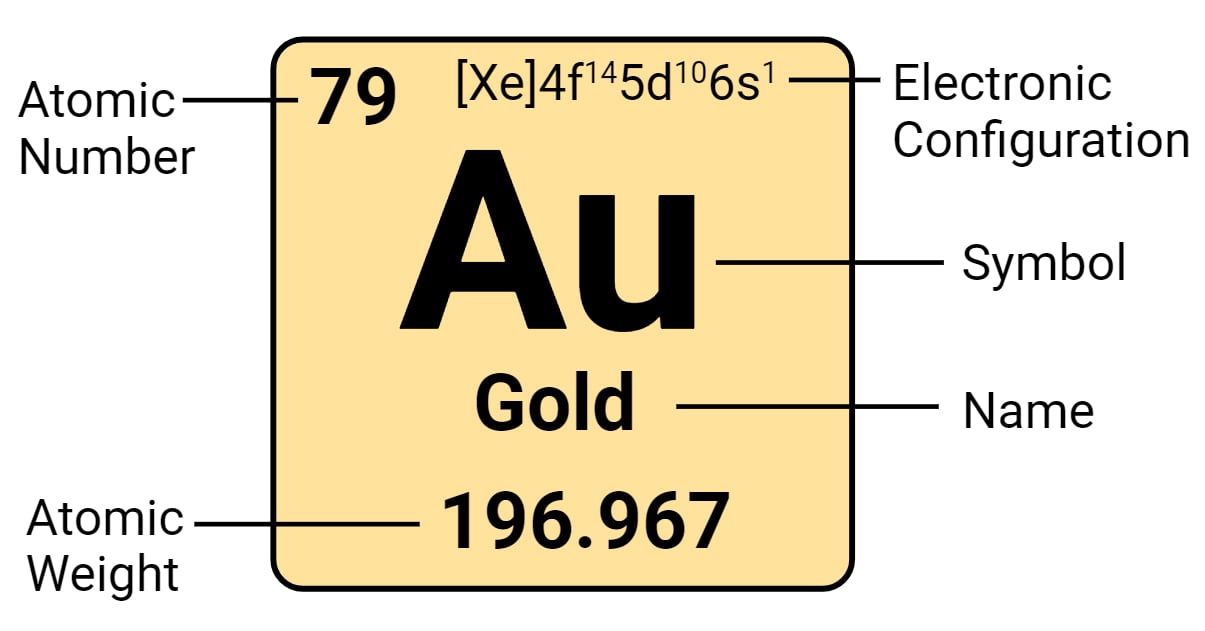
The atomic number of gold is 79. It is found in Group 11 and Period 6 of the periodic chart. The symbol ‘Au’ represents it. The name is derived from the Anglo-Saxon name, while the symbol is derived from the Latin name. Gold is frequently found in its free elemental (native) form as nuggets or grains in rocks, veins, and alluvial deposits. It is found in a solid solution series with the native element silver (as in electrum), naturally alloyed with other metals such as copper and palladium and in mineral inclusions such as pyrite. It may also be found in minerals as gold compounds, frequently with tellurium (gold tellurides).
Gold is exceedingly rare, accounting for only 0.004 parts per million of the Earth’s crust. For instance, copper has 50-60 parts per million, This rarity is ultimately what makes gold so desirable. Gold is a valuable metal found on Earth. Its gleaming surface has captivated users since antiquity. People have manufactured coins and jewelry from it, but gold ornamentation is still made today. Gold was a dependable form of wealth thousands of years ago.
Interesting Science Videos
History of Gold
- As far as we know, humans have been utilizing gold for at least 6200 years. Numerous gold-based artifacts dating to between 4500 and 4000 BC have been found in Bulgaria.
- Gold items dating back to 5000 BC have been discovered in Egyptian tombs; gold was actually being pounded into sheets, foil, and wire in Egypt at the time.
- Gold of 98% purity was discovered in Nahal Quanah, Israel’s ancient kingdom, around 6000 years ago.
- Examinations of gold from ancient Egypt show that purification began somewhere around 2500 years ago.
- Gold became the basis of money in many ancient civilizations, and most countries now keep considerable amounts of gold to preserve financial credibility.
- The term ‘gold’ is an Anglo-Saxon word, as is the term for yellow, ‘geolo.’ It is said to have originated from the Sanskrit ‘jval,’ which means ‘to shine.’
- The chemical symbol Au is derived from the Latin word for gold, ‘aurum‘. Aurora was the goddess of the morning light.
Occurrence of Gold
- Most natural compounds have trace amounts of gold. There are roughly 0.012 parts per billion (ppb) of gold in saltwater and 0.02 parts per billion in surface water.
- Its typical concentration in the Earth’s crust, or lithosphere, is roughly 5 ppb, although it may reach quantities of up to 2100 ppb, or 2.1 parts per million (ppm), in some sedimentary rocks.
- A significant part of gold may be discovered in two types of deposits.
- First, there are hydrothermal veins, where gold is combined with quartz and pyrite (fool’s gold), and then there are deposits, which are generally generated by the weathering of gold-bearing rocks.
- At these concentrations, between 20 and 30 tones of stone need to be treated to recover a single ounce of pure gold.
- Therefore, gold can only be extracted successfully where it has been substantially concentrated by natural chemical and physical processes.
- Gold can be discovered in copper and lead deposits, although the amounts can often be tiny.
- It is easily extracted as a byproduct of mining these basic metals. Gold-bearing rock in large enough amounts to be termed an ore is exceedingly rare.
- With mass numbers ranging from 171Au to 205Au, gold has 35 isotopes whose half-lives are known. Gold’s lone stable isotope, 197Au, makes up all naturally occurring gold.
Isotope of Gold
Gold consists of only one naturally occurring isotope: 197Au.
| Isotope | Natural abundance (atom %) |
|---|---|
| 197Au | 100 |
Elemental Properties of Gold
| Electronic Configuration | [Xe] 4f14 5d10 6s1 |
| Atomic Number | 79 |
| Atomic Weight | 196.9655 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 11, 6, d-block |
| Density | 19.32 g/cm3 at 20 °C |
| Ionic radius | 0.137 nm (+1) |
| Van der Waals radius | 166 pm |
| Electron shells | 2, 8, 18, 32, 18, 1 |
| Electrons | 79 |
| Protons | 79 |
| Neutrons in most abundant isotope | 118 |
Physical Properties of Gold

- Gold has an atomic number of 79 and is a bright yellow metal. It has a melting point of 1064.18°C (1947.52°F) and a boiling point of 2970°C (5378 °F).
- Au has a solid phase density of 19.3 g/cm3 and a liquid or molten phase density of 17.31 g/cm3.
- Au has the highest malleability of any metal; meaning it can be easily hit into sheets without any cleavage.
- It is also ductile metal which can be drawn into thin wires without breaking it.
- Au serves as an excellent electrical conductor. Because electrons in gold are free to move around they are able to carry electrical charge from one end to other.
- Au is an effective thermal conductor as well. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- Both rust as well as corrosion do not affect gold’s exceptional durability.
| Color/physical appearance | Lustrous, reddish-yellow |
| Melting point/freezing point | 1337.33 K (1064.18°C, 1947.52°F) |
| Boiling point | 3243 K (2970°C, 5378°F) |
| Density | 19.3 g/cm3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 2.54 (Pauling Scale) |
Chemical Properties of Gold
- The oxidation states of gold compounds range from -1 to +5.
- It is resistant to ozone attacks up to 100°C.
- Fluorine readily corrodes gold.
- Aqua-Regia, a 1:3 combination of nitric acid and hydrochloric acid, dissolves gold.
- At any temperature, gold does not react with oxygen.
- Most acids, including sulfuric, hydrochloric, hydrobromic, hydroiodic, hydrofluoric, nitric acid, and selenic acids, do not cause gold to react.
Chemical Reaction of Gold
- The Reaction of Gold with Air
This metal is stable in the air under typical circumstances. However, it dissolves in aqueous cyanide solutions in the presence of air.
- The Reaction of Gold with Water
It does not react with water.
- The Reaction of Gold with Halogens
This metal interacts with chlorine, Cl2, to generate the trihalides gold(III) chloride, AuCl3.
2 Au (s) + 3 Cl2 (g) → 2 AuCl3 (s)This metal interacts with bromine, Br2, to generate the trihalides gold(III) bromide, AuBr3.
2 Au (s) + 3 Br2 (g) → 2 AuBr3 (s)This metal interacts with iodine, I2, to generate the monohalide gold(I) chloride, AuI.
2 Au (s) + I2 (g) → 2 AuI (s)- The Reaction of Gold with Acid
Aqua-Regia, a 1:3 combination of nitric acid and hydrochloric acid, dissolves it.
Au (s) + 6 H+(aq) + 3 NO3−(aq) + 4 Cl−(aq) → [AuCl4]−(aq) + 3 NO2 (g) + 3 H2O (l)Uses of Gold
- It is utilized to create jewelry and other artistic products.
- The contacts on computer processors have a gold coating to prevent corrosion.
- As well as being utilized in dentistry, it is also employed in cancer treatments.
- It is frequently plated on top of other metals to give them shine and prevent corrosion.
- It is possible to create a gold thread and use it for embroidery.
- The windows of a big structure have just a bit of gold on them to reflect the heat from the sun.
- To make glass red or purple, colloidal gold is added.
Health Effects of Gold
- If exposure is prolonged or severe, it could irritate. No negative consequences are anticipated after ingestion.
- Skin: Can irritate and trigger allergic responses.
- Eye: Potentially irritating.
- Rheumatoid arthritis, often known as RA, is treated using chromotherapy, a gold-based therapy. It is administered when non-steroid anti-inflammatory medicine is ineffective.
Environmental Effects of Gold
- Gold’s ecotoxicity has not been researched. There is no evidence that gold released into the ecosystem creates ecological issues, and it is predicted that gold’s biodegradation will be fairly poor under aerobic conditions. The bioavailability and biological accumulation properties of gold are assumed to be negligible since it is insoluble.
Video Reference
References
- https://pilgaardelements.com/Gold/Reactions.htm
- https://www.webelements.com/gold/chemistry.html#:~:text=Gold%20metal%20reacts%20with%20chlorine,(I)%20chloride%2C%20AuI.
- https://www.gov.nl.ca/iet/mines/publicoutreach/minerals/gold/
- https://collegedunia.com/exams/gold-occurrence-uses-physical-and-chemical-properties-chemical-articleid-1769
- https://science4fun.info/gold/
- https://www.lenntech.com/periodic/elements/au.htm
- https://www.chemicool.com/elements/gold.html
- https://chemicalengineeringworld.com/gold-element-properties-and-information/


The graphic says 74, not 79.
Thanks, i will correct in the graphics.