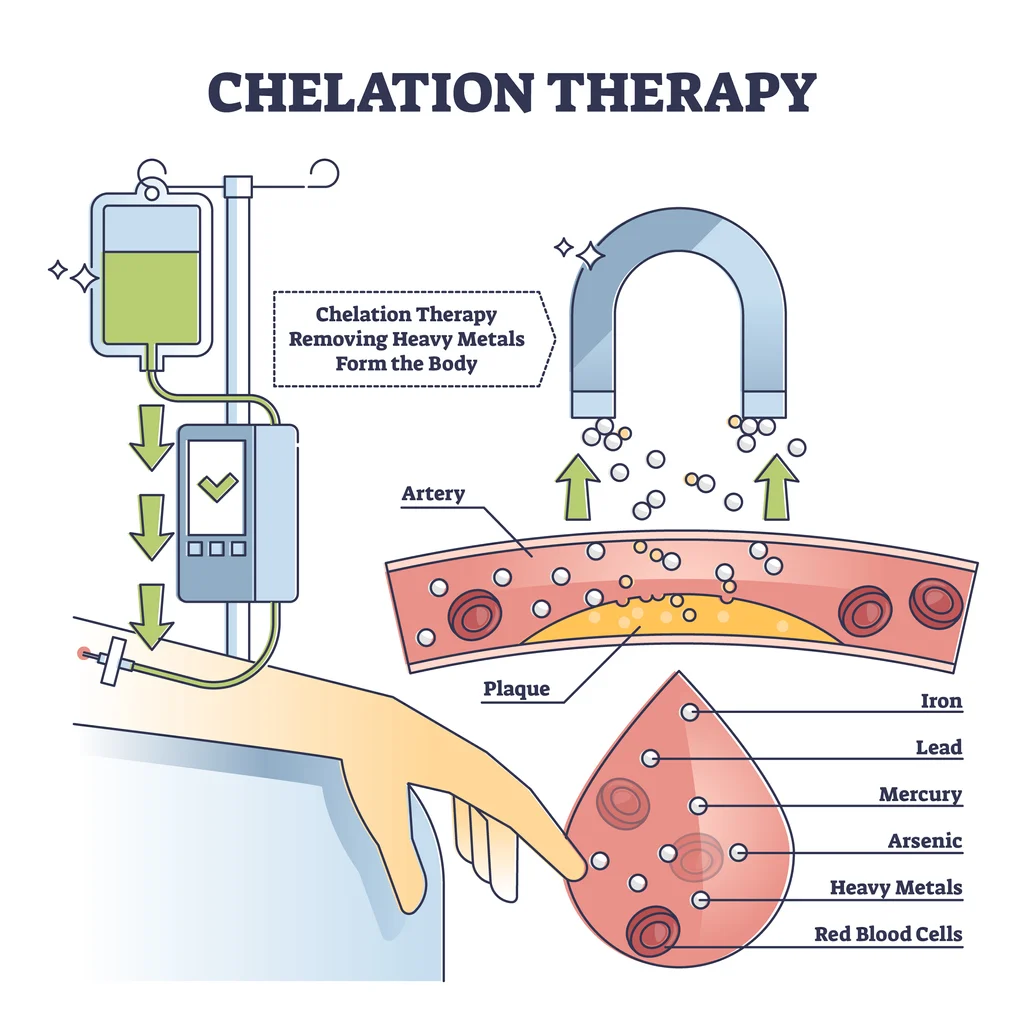
One approach for treating the majority of heavy metal poisonings is chelation therapy. A metal-specific chelating agent, often known as an antidote, is given to the patient orally, intramuscularly, or intravenously. Chelation therapy is a technique used to remove heavy metals from blood, such as lead or mercury. Some additional ailments, including diabetes, heart disease, autism, and Alzheimer’s disease, have reportedly been treated with chelation therapy in recent years, according to some reports.
Dimercaprol (BAL, British anti-Lewisite), calcium disodium edetate, and penicillamine are the three most common chelating agents used in the treatment of heavy metal poisoning. Meso-2,3-dimercaptosuccinic acid, or Succimer, is an unsupervised medication that is effective in treating lead, arsenic, and mercury poisoning, among other heavy metal poisonings. These and other chelating compounds, like α-lipoic acid (ALA) and unithiol (DMPS, 2,3-dimercapto-1-propanesulfonic acid), are also utilized in alternative medicine, which has generated a lot of debate and controversy. Chelation therapy has not yet been shown to be beneficial in any clinical setting aside from heavy metal intoxication.
Chelation therapy is a particularly successful treatment for arsenic, mercury, and lead poisoning. Cadmium poisoning is extremely difficult to treat, and no truly effective treatment has been discovered yet.
Interesting Science Videos
How does it work?
The mechanism of action relies on the chelating agent’s capability to attach to metal within the body tissues, creating a chelate. Subsequently, this compound is liberated from the tissues and circulates in the blood. Ultimately, the kidneys filter the complex, which is then expelled through urine. Regrettably, this procedure mandates hospitalization due to potential discomfort, necessitating the stabilization of the patient’s vital functions. Moreover, the patient may need interventions for complications linked to heavy-metal poisoning, such as addressing anemia, kidney failure, or shock reactions.
Some of the common chelating agents used in the treatment of heavy metal poisoning are:
Calcium disodium edetate
- Calcium disodium edetate, also known as calcium sodium EDTA, represents the chemical compound 2,2′,2′′,2′′′-(ethane-1,2-diyldinitrilo)tetraacetic acid, categorized as a synthetic amino acid.
- Edetate denotes the calcium disodium salt of the chelating agent with the formula (HO2CCH2)2NCH2CH2N(CH2CO2H)2.
- Edetate is primarily employed to form complexes with di and trivalent metal ions. It can attach to metals through its four carboxylate and two amine groups, creating particularly robust complexes with Co(III), Cu(II), Mn(II), and Fe(III).
- Lead poisoning, which was particularly problematic for navy troops following World War II, can be treated with edetate.
- When lead-based paint was used to repaint navy ships, workers were exposed to heavy metals and experienced lead poisoning symptoms. Edetate was first used as a chelating agent in medicine at this period.
- Among the adverse effects are stomach pain, diarrhea, and nausea. If used in excess, it may cause harm to the kidneys.
- Nevertheless, compared to the majority of other chelating drugs in use, these adverse effects are less severe and intrusive.
- Usually, edetate is infused intravenously over a maximum of five days.
- Utilizing chromium-EDTA to assess kidney function is one of the further clinical applications. It is injected intravenously, and the monitoring of its filtration into the urine is done. The only method that chromium-EDTA leaves the human body is by glomerular filtration; it is not released or processed in any other manner. EDTA and its salts are frequently included as additions to blood collection bottles because they can function as an anticoagulant for blood samples.
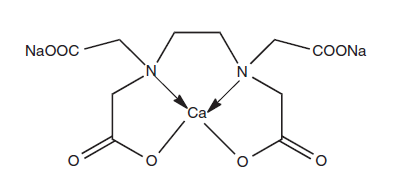
Chemical structure of edetate
Dimercaprol (BAL)
- A chelating compound called dimercaprol (BAL) is used to treat poisoning from arsenic, antimony, bismuth, gold, and mercury.
- 2,3-dimercapto-1-propanol is the chemical name for it, and it is a clear, colorless, or slightly yellow liquid.
- During World War II, BAL was also created by British researchers at the University of Oxford as a Lewisite countermeasure. Lewisite is a chemical warfare agent with an arsenic basis that is applied as blister gas. It can be employed as an antidote against a range of harmful metals, according to more studies.
- Wilson disease, a chronic condition in which the body accumulates excessive levels of copper, was treated with it.
- The coordination of the metal to sulfhydryl groups of enzymes, which implies that these enzymes are prevented from functioning, is frequently the cause of heavy metal poisoning. BAL essentially competes with the enzymes for the coordination of the metal because it also has sulfhydryl groups. After that, the chelated complex is eliminated in the urine. Although BAL eliminates a variety of heavy metals, its usage is constrained since it also appears to raise the concentration of other metals in the human body. It is not recommended as an antidote for iron poisoning, selenium, or cadmium poisoning (increased amounts are discovered in the kidneys following therapy).
- However, BAL has a limited therapeutic window and is highly toxic. Its numerous adverse effects include but are not limited to, tachycardia, nausea, vomiting, burning sensation in the muscles, dizziness, and hypertension. It is administered via intramuscular injection, and it hurts a little bit. It is made with peanut oil as a solvent due to its volatility in water.
Chemical structure of dimercaprol
Dimercaptosuccinic acid (DMSA)
- DMSA is a derivative of BAL that has two carboxylic acid groups and two thiol groups, which are in charge of the off-putting odor.
- DMSA is sometimes referred to as Succimer. Its chemical formula is HO2CCH(SH)CH(SH)CO2H, and its name is meso-2,3-dimercaptosuccinic acid. Meso and chiral DL forms are the two diastereomeric forms; the meso isomer is employed as a chelating agent.
- When it came to treating lead, arsenic, and mercury poisoning, DMSA, which was created in the 1960s, took the role of BAL and edetate in several nations. Moreover, heavy-metal toxicity has been effectively treated using DMSA’s dimethyl ester modification.
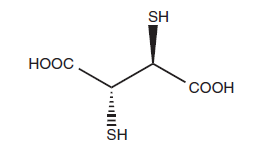
Chemical structure of DMSA
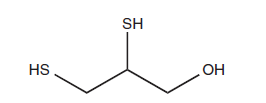
2,3-Dimercapto-1-propanesulfonic acid (DMPS)
- Another chelating agent that contains thiols is DMPS.
- It also has an extra sulfate group and sulfhydryl groups. Researchers from the former Soviet Union discovered that DMPS functions as a helpful chelating agent and can counteract mercury in certain cases.
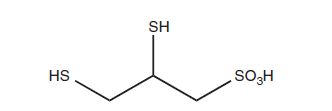
Chemical structure of DMPS
Lipoic acid (ALA)
- Lipoic acid, with the formula C8H14S2O2 and name 5-(1,2-dithiolan-3-yl)pentanoic acid, is also referred to as α-lipoic acid (alpha-lipoic acid) or thioctic acid. It has a disulfide group that the body can change into a dithiol group.
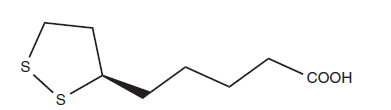
Chemical structure of ALA
Proven benefits of chelation therapy
Chelation therapy is a highly efficient method of removing several heavy metals from blood, such as:
- Lead
- Arsenic
- Mercury
- Iron
- Copper
- Nickel
Unproven benefits of chelation therapy
- Heart disease: Chelation therapy is regarded as a treatment for atherosclerosis, a condition in which plaque accumulates in the arteries. It may eventually result in heart disease. Chelation agents, according to their proponents, bind to the calcium in plaque to help dissolve and release the accumulation. Although this makes sense, there isn’t much proof that chelation therapy is beneficial. For instance, there was insufficient data from a large-scale clinical trial. The source included individuals who had already experienced a heart attack to justify the regular use of chelation therapy for heart disease. Even though some participants experienced a lower risk of developing additional heart issues, this was insufficient to outweigh the associated dangers, which we addressed later.
- Alzheimer’s disease: Based on the theory that metal builds up in the brain via aluminum food, drink, pots and pans, and deodorant, chelation therapy is used to treat Alzheimer’s disease. Even so, other researchers disagree that there is any connection between aluminum exposure and Alzheimer’s disease, according to a review of previous studies. The majority of chelators are too big to pass through the blood-brain barrier, regardless of how the two are related. This barrier regulates what goes into and comes out of your brain like a kind of net. Though it hasn’t been verified, some experts think that EDTA might be able to penetrate the brain.
- Diabetes: Sugar chelation is not a diabetes treatment. However, the likelihood of developing cardiac issues is significantly increased in individuals with diabetesTrusted Source. Chelation treatment could lessen this danger. According to Trusted Source, EDTA did lower the risk of cardiac issues in diabetics, but not in non-diabetics. Even though these preliminary results are encouraging, several larger clinical trials including individuals with diabetes are required.
- Parkinson’s disease: It is well known that iron accumulates in the brains of Parkinson’s patients. Nevertheless, the part iron plays in the illness is still not entirely understood by researchers. Furthermore, it’s unclear if iron removal from the brain helps Parkinson’s sufferers in any way.
References
- Katjia A. Strohfeldt, Essential of Inorganic Chemistry
- https://www.webmd.com/balance/what-is-chelation-therapy
- https://www.healthline.com/health/chelation-therapy
- https://vascular.tcvcg.com/blog/does-chelation-therapy-work-to-get-rid-of-the-plaque-in-my-arteries
