
Chemical lab equipment is an important component of every chemical lab. Experimenting without it would be difficult, if not impossible. There are numerous types of laboratory equipment available, each with a distinct purpose. Different types of Chemical lab equipment include:
Interesting Science Videos
Different Chemical lab equipment
Laboratory balances
In general, laboratory balances assess the mass of an object; in the laboratory, they are used to measure solids, liquids, and tissue. They have a wide range of applications in practically any laboratory, including clinical, scientific, and environmental settings. Triple beam balances, analytical balances (also known as precision balances or chemical balances), and micro and semi-micro balances are types of laboratory balances. These balances may weigh anything from a single grain of a chemical solid on a microbalance to the weight of a huge beaker on a triple beam balance.
laboratory balances
Balances have a high degree of readability, a wide weighting range, and a high level of precision. However, the laboratory atmosphere, operational temperature, moisture, vibration, and ventilation current can all have an impact on the outcome. As a result, it is critical to keep the weighing pan enclosed or sealed to avoid contact with dust or other pollutants. To avoid the formation of air currents inside the enclosed space, samples are kept at ambient temperature. Additionally, ensure that the balance is kept clean, exactly leveled, and regularly maintained.
Bunsen Burner
The Bunsen burner is the most commonly associated object with a chemistry laboratory.
It is the principal heat source in this lab. The Bunsen burner is a type of gas burner invented in 1857 by a German chemist named Robert Bunsen. It generates a smokeless, non-luminous flame that is essential to start certain chemical reactions. The flame is created by a mixture of gases such as methane, ethane, propane, and others. These gases are known as natural gases, and they can be adjusted by altering the Bunsen burner.
Bunsen burner
Depending on your gas source and experimental settings, there are numerous varieties of Bunsen burners. Some of them are:
Meker burner
The Meker burner is a type of burner that produces a very hot and consistent flame. The barrel is larger in diameter. Because of the larger size, there is more mixing of air and gas. A grid is placed over the top of the barrel to divide the flame into smaller flames. To control the flow of gas, a gas valve located beneath the chimney or barrel might be employed.
Tirrill burners
Tirrill burners are a type of Bunsen burner with a wing nut at the bottom of the tube to control the gas flow. Turning this valve allows for minor gas adjustments.
Micro Bunsen burners
Micro Bunsen burners are miniature burners that are utilized in smaller laboratory settings or for more sensitive tasks.
Fisher burner
This model is intended for usage with natural gas. It is frequently used in analytical chemistry.
Teclu burner
This burner generates more heat more efficiently. In comparison to other burners, it has a longer barrel tube. The air and gas have been fully combined. As a result, the combustion power of the flame increases. The barrel tube also features a screw nut for regulating the gas supply.
Centrifuge
A centrifuge is a laboratory apparatus used to separate fluids or liquids, based on density. Separation is accomplished by rapidly spinning the container containing the material.

Centrifuge
A centrifuge operates on the idea of gravitational force sedimentation. When a tube is spun with the bottom side facing the spin, the centrifugal force caused by the spin acts on the sample in the tube-like high gravity. This causes the heavier components of a mixture to descend quicker than the lighter components, and they neatly stack at the bottom of the tubes.
Fume Hood
A fume hood is a ventilated enclosure that captures and removes gases, vapors, and fumes from the work area. An exhaust fan on the roof of the laboratory building draws air and airborne contaminants through attached ductwork and exhausts them into the atmosphere.
The laboratory chemical fume hood is the most frequent local exhaust ventilation system used in laboratories and is the major approach for controlling hazardous substance inhalation exposures. Fume hoods provide great safety for the user when used properly.

The goal of a chemical fume hood is to manage and then exhaust hazardous and/or odorous compounds to prevent their release into the general laboratory space. If an unintentional spill occurs, the fume hood will confine the chemicals and exhaust the fumes away from the user and laboratory zone.
Laboratory hot plates
Laboratory hot plates are crucial in scientific research and healthcare for heating and boiling liquids. These devices provide a regulated heat source for performing chemical processes, sterilizing equipment, and other tasks.

Because of technical improvements, different varieties of laboratory hot plates are now available on the market, each tailored for a unique application. There are different types of hot plates, they are:
Aluminum hot plates
Aluminum hot plates are normally affordable and can operate at high temperatures. However, with frequent usage, they may wear out rapidly and require occasional cleaning to avoid leaving a residue on the surface. It delivers more heat and is more durable.
Ceramic hot plates
Ceramic hot plates are constructed of porcelain and can tolerate high temperatures without causing surface damage. As a result, they can last a long period and may not require cleaning. The surface is suitable for heating liquids or other things that must be kept away from aluminum or other metals.
Magnetic Stirring hot plates
Magnetic Stirring hot plates have an extra stirring magnet incorporated into the top plate to mix liquids as they heat up on the surface below. Some Laboratory Hotplates with Magnetic Stirrers can automatically regulate their heating element to maintain a steady temperature over time.
Stainless steel hot plates
These are built of high-quality materials and can resist the most extreme operating temperatures. As a result, they often survive longer than other types of hot plates, but they may be more expensive to purchase at first. They may also require specialized cleaning chemicals or compounds.
Stirring hot plate
The heating and stirring elements are situated beneath the machine’s flat top surface in this hot plate. Unlike other types of stirring hot plates, these lack a top-surface clamp mechanism and must be fastened to the bottom surface with a separate magnetic bar or clamp.
Microscope
Microscope, an equipment that magnifies images of small objects, allows the observer to see minute structures at a scale suitable for investigation and analysis.
Microscope
two main types of microscope are:
Light microscope
These are basic microscopes that magnify objects using light. These microscopes’ lenses refract light, making the objects beneath them look closer.
Electron microscope
Instead of light, electron beams are used to generate images in these microscopes.
Spectrophotometer
To perform qualitative and quantitative examination of the substance, the spectrophotometer measures the absorbance of the tested substance at a specified wavelength or within a specific wavelength range. The fundamental principles of several types of spectrophotometers are identical.
Spectrophotometer
They all use a light source that can create many wavelengths to form a light source of a specific wavelength via a succession of light-splitting devices. Part of the light source is absorbed after it passes through the test sample. Following that, by measuring the absorbance of the sample, the concentration of the sample can be calculated. The absorbance of the sample is proportional to its concentration.
VIS spectrophotometer
It is a device that measures the absorbance of a substance to visible light and performs qualitative and quantitative analysis on it.
UV VIS spectrophotometer
A UV VIS spectrophotometer is an analytical equipment that analyses radiation absorption in the ultraviolet-visible spectrum region by material molecules. This equipment is used to determine the sample’s absorbance to visible or ultraviolet light.
Infrared spectrophotometer
The light emitted by the light source is precisely described by an infrared spectrophotometer, which is separated into two beams of equal energy and symmetry. One beam is the sample light flowing through the sample, and the other beam serves as the reference light. As this is the most extensively studied spectral area for organic compounds, it can be used to analyze samples in a variety of states (gas, liquid, and solid).
Fluorescence spectrophotometer
A fluorescence spectrophotometer scans the fluorescence spectrum generated by liquid-like fluorescent markers. It may give a variety of physical parameters, such as the excitation spectrum, emission spectrum, fluorescence intensity, quantum yield, fluorescence lifetime, fluorescence polarisation, and so on, that can reflect the bonding and structure of molecules from various viewpoints.
Spring balance
Spring balance is a weighing device that uses the relationship between applied weight and spring deformation. This relationship is normally linear; if the load is doubled, so is the deformation. It operates on Hooke’s Law concept, which asserts that the force required to extend a spring is proportional to the distance the spring is extended from its rest position.
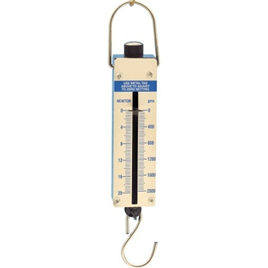
Spring balances are a simple and inexpensive way of measuring mass. The mass is suspended from the end of a spring, and the deflection of the spring caused by the mass’s downward gravitational pull is measured against a scale. Because spring features are subject to environmental changes, measurement accuracy is typically low.
pH strips
pH strips are used to determine whether a solution is acidic or alkaline. The strips are immersed in the solution, and their colors are compared to a color chart to establish the pH. pH strips are used for a variety of purposes, including evaluating the pH of swimming pools, aquariums, and soil.

pH strips
They are also useful for determining the acidity of food and beverages. The pH of a solution is essential because it can indicate whether or not a chemical is corrosive. As a result, it is critical to test solutions using pH strips before coming into touch with them.
chemical lab equipment is an important component of every chemical lab. Experimenting without it would be difficult, if not impossible. There are numerous types of laboratory equipment available, each with a distinct purpose.
References
- https://en.seamaty.com/index.php?s=/sys/518.html
- https://www.edrawsoft.com/laboratory-equipment-shapes.html
- https://chem.libretexts.org/Courses/Hope_College/General_Chemistry_Labs/Lab_Equipment
- https://us.vwr.com/cms/types_of_lab_hot_plates
- https://www.purdue.edu/ehps/rem/laboratory/equipment%20safety/Lab%20Safety%20Equipment/cfh.html
- https://scienceinfo.com/chemistry-lab-glassware/
