
Chloroform is a colorless and volatile liquid. It is a trichloromethane derivative with an ether-like odor. It is represented by the chemical formula CHCl3. The hydrogen bonded to the carbon in chloroform contributes to hydrogen bonding.
Many different types of seaweed produce chloroform, and fungi are also thought to produce chloroform in soil, making them natural sources of chloroform. Dr. Samuel Guthrie, an American chemist, synthesized chloroform in 1831 by combining whisky with chlorinated lime in an attempt to create an inexpensive insecticide.
It is commonly used as an organic solvent. It is employed in the synthesis of several chemical substances. In the medical field, chloroform is often used for anesthesia.
Interesting Science Videos
Structure of chloroform
Chloroform is an organic molecule containing three halogen atoms linked to a carbon atom. The compound has the formula CHCl3. The center carbon atom is sp3 hybridized.
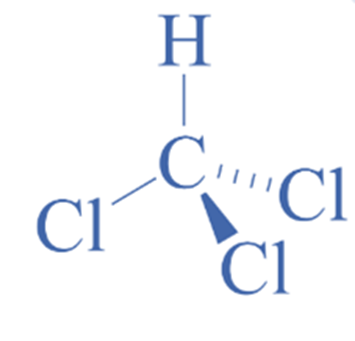
The VSEPR theory, or Valence shell electron pair repulsion hypothesis, can be used to determine the form and geometry of a molecule. According to the hypothesis, the number of bonding and lone pairs of electrons around the core atom determines the structure of any molecule. The compound adopts the most stable form with the least repulsion between distinct groups. If a center atom has four linked atoms and no lone pair, the general formula is AX4, and the molecule’s geometry is tetrahedral.
As a result, the molecular geometry of CHCl3 is tetrahedral since it has four bonded atoms and no lone pair with sp3 hybridization.
Preparation of chloroform
From methane
Chloroform can be prepared by chlorination of methane at 400 oC.
CH4 + Cl2 → CH3Cl + CH2Cl2 + CHCl3 + CCl4
Chloroform is separated by fractional distillation.
From chloral hydrate
Pure chloroform is produced by distilling a mixture of chloral hydrate and a strong solution of sodium or potassium hydroxide.
NaOH + CCl3CH(OH)2 → CHCl3 + HCOONa + H2O
Laboratory preparation
Principle
Chloroform is produced in the laboratory by heating ethyl alcohol or acetone with bleaching powder and water. In this reaction, firstly, chlorine is produced by the reaction of water with bleaching powder.
CaOCl2 + H2O → Ca(OH)2 + Cl2
a. From Ethanol
i. Oxidation of Ethanol
C2H5OH + Cl2 → CH3CHO + 2 HCl
ii. Chlorination of Ethanal
CH3CHO + 6 Cl2 → CCl3CHO + 3 HCl
iii. Hydrolysis of Chloral
2 CCl3CHO + Ca(OH)2 → 2 CHCl3 + (HCOO)2Ca
b. From Acetone
i. Chlorination of Acetone
CH3COCH3 + 3 Cl2 → CCl3COCH3 + 3 HCl
ii. Hydrolysis
2 CCl3COCH3 + Ca(OH)2 → 2 CHCl3 + (CH3COO)2Ca
Procedure
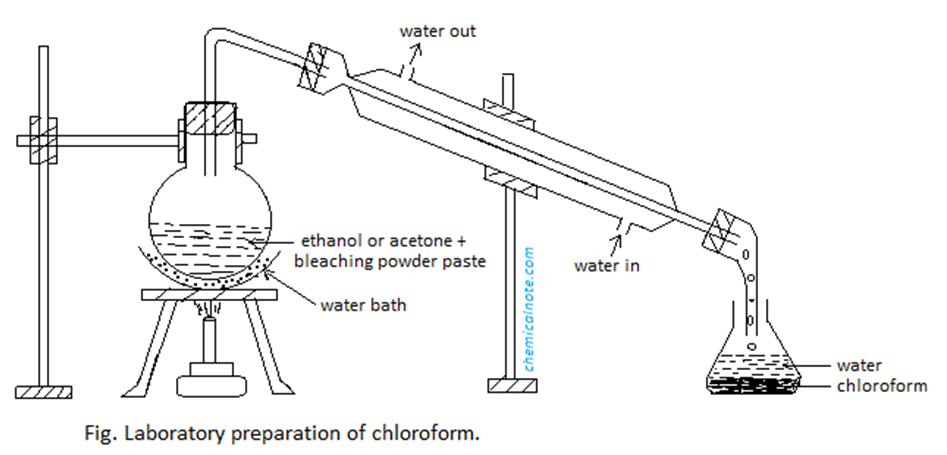
Image source: https://chemicalnote.com/chloroform-lab-preparation-properties-uses-and-question-answer/
In an R. B. flask, a mixture of bleaching powder, water, and ethanol or acetone is taken. Then, It is gradually heated in a hot water bath. The condenser condenses the distillate of chloroform. Chloroform that has been condensed is collected in the receiver.
Purification
In order to neutralize the mixture’s acidity, the chloroform is rinsed with sodium hydroxide (NaOH) using a separating funnel. It is then cleaned with water. After washing, it is dried and redistilled at temperatures 60°C to 65°C. Chloroform is obtained in its purest and most dry form.
Industrial preparation
Chloroform can be produced in large quantities by heating a mixture of ethyl alcohol or ketones such as acetone, water, and bleaching powder in the same way that it is produced in laboratories. In another approach, carbon tetrachloride is partially reduced by iron powder and water vapor to increase its quantity.
3Fe + H2O → Fe3O4 + 8H
CCl4 + 2H → CHCl3 + HCl
Physical properties of chloroform
- Chloroform is a colorless volatile liquid.
- The odor of chloroform is pleasant and nonirritating
- its molecular weight is 119.38 g/mol.
- It has a boiling point of 61°C.
- It is nearly insoluble in water but soluble in ether and alcohol.
- As it is heavier than water, it generates a lower surface when mixed with it.
- It is an excellent solvent for oils, fats, waxes, rubber, and other similar substances.
- It is not flammable, however, its vapors can ignite with a green flame.
- When enough vapors are inhaled, it causes temporary unconsciousness.
Reactions of chloroform
Oxidation
In the presence of sunlight and air, chloroform progressively oxidizes to create carbonyl chloride i.e., phosgene.
2 CHCl3 + O2 → 2 COCl2 + 2 HCl
Phosgene is an extremely poisonous gas. To prevent oxidation, chloroform is stored in blue or brown bottles that absorb sunlight through the colored glass.
Stoppers must be used on the mouths of these bottles to prevent chloroform from being exposed to excessive air.
1% ethyl alcohol is also added to protect the chloroform from oxidation. Ethyl alcohol works as a negative catalyst for this oxidation reaction. It also interacts with phosgene to generate the nontoxic ethyl carbonate.
COCl2 + 2 C2H5OH → (C2H5)2CO3 + 3 HCl
Reduction
In the presence of ethanol, Chloroform reacts with zinc and hydrochloric acid to form dichloromethane. Zinc and diluted HCl react to produce the nascent hydrogen required in this synthesis.
CHCl3 + 2 (H) → CH2Cl2 + HCl
Hydrolysis
It produces a salt of formic acid when heated with a strong alkali aqueous or alcohol solution. For example, the reaction of chloroform with sodium hydroxide, produces sodium formate, sodium hydroxide, and water.
CHCl3 + 4 NaOH → HCOONa + 3 NaCl + 2 H2O
Reaction with silver powder
Chloroform on heating with silver powder produces acetylene (ethyne).
2 CHCl3 + 6 Ag → HC ≡ CH + 6 AgCl
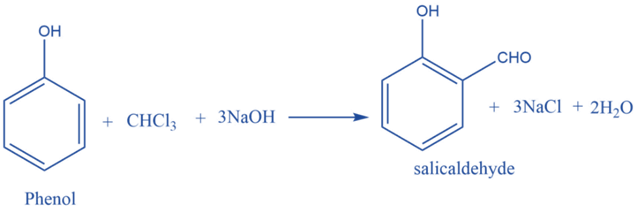
Reimer Tiemann reaction
Chloroform on heating with phenol in the presence of a base, Produces salicylaldehyde. This is known as the Reimer-Tiemann reaction.
Carbylamine reaction
In the presence of alcoholic KOH, primary amines react with chloroform to give isocyanides, also known as carbylamine. These have an offensive smell. This reaction is known as the carbylamine reaction.
CH3NH2 + CHCl3 + 3 KOH → CH3NC + 3 KCl + 3 H2O
C6H5NH2 + CHCl3 + 3KOH → C6H5NC + 3 KCl + 3 H2O
As secondary and tertiary amines do not respond to this reaction, it is employed as a test reaction for primary amines.
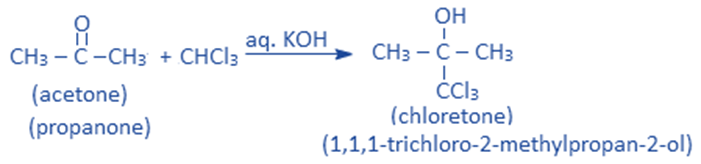
Reaction with acetone
In the presence of a base like aq. KOH, chloroform reacts with acetone to give 1,1,1-trichloro – 2 – methylpropane – 2-ol ie., chloretone. Chloretone is a sleep-inducing (hypnotic) drug.
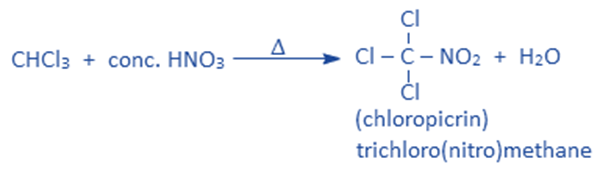
Reaction with HNO3
Chloroform on heating with conc. HNO3 produces chloropicrin. Chloropicrin is used as an insecticide and tear gas.
Chlorination reaction
In the presence of sunlight, Chloroform reacts with chlorine to form carbon tetrachloride. Carbon tetrachloride is a solvent in the chemical and pharmaceutical industries, as well as an occluding and nonoxidizing agent in fire extinguishers.
CHCl3 + Cl2 → CCl4 + HCl
Uses of chloroform
- It is used in laboratories as a reagent.
- It is also used in the construction, paper, and board industries, as well as pesticide and film manufacturing.
- It also plays an important part in the manufacture of the refrigerant Freon.
- Other applications include lacquers, floor polishes, resins, adhesives, alkaloids, fats, oils, and rubber.
- It is also used as an anesthetic in dentistry during root canal treatments.
- It is used in agriculture as a solvent.
- It’s a type of anesthesia. As very minor excesses are highly poisonous, its use as an anesthetic is discouraged.
- It’s a solvent for dyes and insecticides.
- It is used to preserve dead bodies and anatomical specimens.
- It is used in pharmaceuticals.
- Chloroform was once used as an extraction dissolvable for fats, greases, oils, and other things; also as a laundry spot.
- It is used as an indirect food additive in food packaging materials for adhesive components and as a part of food contact products.
- The most common solvent used in Nuclear Magnetic Resonance (NMR) spectroscopy is chloroform containing deuterium (heavy hydrogen), CDCl3.
Sources and Potential Exposure
- Chloroform may be discharged into the environment from a variety of sources, including its production and usage, as well as its generation during the chlorination of drinking water, wastewater, and swimming pools.
- As a result, It can also be found in wastewater from sewage treatment plants and drinking water having added chlorine. This Chlorination of water is being done to destroy bacteria.
- Chloroform enters the environment through chemical plants and paper mills. Chloroform enters the environment from chemical industries and paper mills. Chloroform can also be found in various foods, beverages, and tap water.
Health hazards of chloroform
- Changes in respiration rate, cardiac effects, gastrointestinal effects such as nausea and vomiting, and effects on the liver and kidney have all been observed in persons exposed to chloroform during anesthesia.
- Chronic inhalation of chloroform in humans has been linked to liver effects such as hepatitis and jaundice, as well as central nervous system effects such as depression and irritability.
- Chronic chloroform exposure can cause dizziness, tiredness, drowsiness, memory loss, increased dreams, anorexia, and palpitations.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992.
- https://byjus.com/chemistry/chloroform/
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- https://chemicalnote.com/chloroform-lab-preparation-properties-uses-and-question-answer/.
- https://www.vedantu.com/chemistry/chloroform
