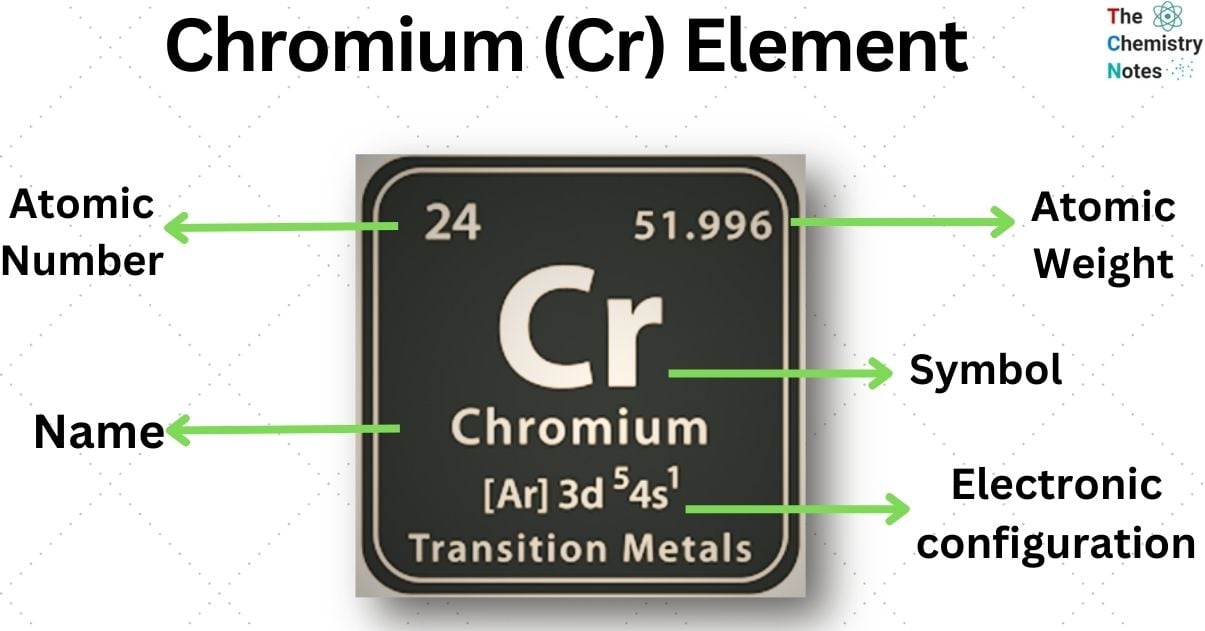
Chromium is a chemical element with the atomic number 24 and it is represented by the symbol ‘Cr’ in the periodic table. It is the first element of the group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Although chromium is the 21st most abundant element in the Earth’s crust, it is not found in its free metal form. Instead, it is mostly found in chromite ore. The sodium and potassium chromates, dichromates, and potassium and ammonium chrome alums are among the most significant chromium compounds.
Interesting Science Videos
History of Chromium
- German mineralogist and geologist Johann Gottlob Lehmann discovered an orange-red mineral in the ore mines of the Ural Mountains on July 26, 1761, and called it Siberian red lead (It is now known as crocoite (PbCrO4) and is a form of lead chromate).
- In the year 1770, Prussian zoologist and botanist Peter Simon Pallas visited the same site as Lehmann and found a red lead mineral that was discovered to possess useful properties as a pigment in paints.
- In 1797, French chemist Louis-Nicolas Vauquelin discovered chromium while working with a substance known as Siberian red lead (mineral crocoite (PbCrO4)).
- 1798 was the year when Louis-Nicolas Vauquelin was able to obtain metallic chromium by simply heating chromium oxide Cr2O3 in a charcoal oven.
- He named it chromium after the Greek word chroma, which means color, as he was intrigued by the diversity of colors it was capable of producing in solution.
- In 1798, Louis-Nicolas Vauquelin also discovered Beryllium.
Occurrence of Chromium
- Despite being the 21st most prevalent element in the Earth’s crust, chromium is not found in its free metal form.
- Chromium is mined as the mineral chromite (FeCr2O4).
- Chromium is found in trace amounts throughout the environment and is mined as a mineral chromite, which is generally associated with ultramafic (an igneous rock with an extremely low silica content) and serpentine rocks (metamorphosed residues of magnesium-rich igneous rocks).
- Chromium is also linked with other ore bodies (such as uranium and phosphorites) and may be found in tailings and other beneficiation wastes from these mining activities.
- Acid mine drainage can make chromium accessible to the environment. Chromium and its derivatives are employed in refractories, drilling muds, electroplating cleaning agents, catalytic manufacturing, and the synthesis of chromic acid and speciality chemicals.
- Ores of chromium are now mined in South Africa, Zimbabwe, Finland, India, Kazakhstan, and the Philippines.
Isotopes of Chromium
Naturally occurring Chromium (Cr) is composed of four stable isotopes, 50Cr, 52Cr, 53Cr, and 54 Cr.
| Isotope | Natural abundance (atom%) |
|---|---|
| 50Cr | 4.345 (13) |
| 52Cr | 83.789 (18) |
| 53Cr | 9.501 (17) |
| 54Cr | 2.365 (7) |
Elemental Properties of Chromium
| Electronic Configuration | [Ar]3d5 4s1 |
| Atomic Number | 24 |
| Atomic Weight | 51.996 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 6, 4, d-block |
| Density | 7.15 g.cm -3 at 20 °C |
| Ionic radius | 0.061 nm (+3) ; 0.044 nm (+6) |
| Van der Waals radius | 0.127 nm |
| Electron shells | 2, 8, 13, 1 |
| Electrons | 24 |
| Protons | 24 |
| Neutrons in most abundant isotope | 28 |
Physical Properties of Chromium
- It has a high melting point of 1,857 °C (3,375 °F) and a boiling point of 2,672 °C (4,842 °F).
- It does not tarnish in air, but when heated, it creates green chromic oxide.
- Chromium compounds are toxic.
- When polished, chromium is a silvery-grey metal with a beautiful luster.
- Chromium reacts passively with oxygen, forming a very thin, insoluble protective oxide that inhibits the underlying metal from further oxidation.
- Chromium, which has the lowest specific gravity among high melting point metals such as tungsten and molybdenum,and is extremely heat and corrosion resistant.
- One of the most essential properties of chromium is that it does not corrode readily, making it ideal for electroplating.
- It is also a good conductor of heat and electricity.
- The chromium sheet is malleable above 500 degrees Fahrenheit and can be easily bent and cut at this temperature, but it is brittle when cold.
- Chromium is paramagnetic, which means that it is weakly attracted to magnetic fields.
| Color/physical appearance | silver-gray |
| Melting point/freezing point | 1907°C, 3465°F, 2180 K |
| Boiling point | 2671°C, 4840°F, 2944 K |
| Density | 7.15 g.cm -3 |
| Malleability | Yes |
| Ductility | No |
| Electronegativity | 1.66 (Pauling Scale) 1.65(Allen Scale) |
Chemical Properties of Chromium
- Chromium is a moderately active metal. It does not react with water, although it does with most acids.
- Chromium is particularly resistant to common corrosive chemicals, which explains its widespread usage as an electroplated protective coating.
- When chromium is heated, it burns and generates a green chromic oxide complex.
- Chromium shields the metal beneath by quickly forming a thin oxide layer that is impenetrable to oxygen.
Chemical Reaction of Chromium
- Reaction of Chromium with air and water
Chromium does not react with air and water at room temperature.
- Reaction of Chromium with Acids
Metallic chromium dissolves in dilute hydrochloric acid, creating Cr(II) and hydrogen gas, H2. Cr(II) exists in aqueous solution as the complex ion [Cr(OH2)6]2+. Similar findings are obtained with sulfuric acid, however pure chromium samples may be resistant to assault. Instead of being dissolved by nitric acid, HNO3, chromium metal is passivated.
Cr (s) + 2HCl (aq) → Cr2+ (aq) + 2Cl– (aq) + H2 (g)
Reaction of Chromium with Hydroxide Ions
Hydroxide ions precipitate Cr(III) as Cr(OH)3. The precipitate has amphoteric properties:
[Cr(H2O)6]3+(aq) [violet] + OH−(aq) ⇌ Cr(OH)3(H2O)3(s) [green] + 3 H2O(l)
Cr(OH)3(H2O)3(s) [green] + 3 OH−(aq) ⇌ [Cr(OH)6]−(aq) [green, octahedral] + H2O(l)
- Reaction of chromium with the halogens
At 400°C and 200-300 atmospheres, chromium combines immediately with fluorine, F2, to generate chromium(VI) fluoride, CrF6.
Cr(s) + 3F2(g) → CrF6(s) [yellow]
Chromium metal interacts with the halogens fluorine, chlorine, bromine, and iodine, to create the trihalides chromium(III) fluoride, CrF3, chromium(III) chloride, CrCl3, chromium(III) bromide, CrBr3, or chromium(III) iodide, CrI3.
2Cr (s) + 3F2 (g) → 2CrF3 (s) [green]
2Cr (s) + 3Cl2 (g) → 2CrCl3 (s) [red-violet]
2Cr (s) + 3Br2 (g) → 2CrBr3 (s) [very dark green]
2Cr (s) + 3I2( g) → 2CrI3 (s) [very dark green]
Chromium(V) fluoride, CrF5, is produced at milder circumstances.
2Cr (s) + 5F2 (g) → 2CrF5 (s) [red]
- Reaction of Chromium with Ammonia
Cr(III) is precipitated as Cr(OH)3 by NH3
[Cr(H2O)6]3+(aq) + 3 NH3(aq) → [Cr(OH)3(H2O)3](s) [green] + 3 NH4+(aq)
Excess ammonia dissolves the precipitate.
[Cr(OH)3(H2O)3](s) + 6 NH3(aq) → [Cr(NH3)6]3+(aq) + 3 H2O (l) + 3 OH−(aq)
Uses of Chromium
Due to its hardness and corrosion resistance, chromium has a broad variety of uses. It primarily serves the metallurgical, chemical, and refractory sectors as well as have other amazing applications.
In Metallurgical Industry
- Chromium is a hard metal that is frequently added to steel to create a hard, corrosion-resistant alloy. This alloy is mostly used to refine stainless steel, heat-resistant steel, and different materials for electric heating elements.
- A thin, solid layer of chromium oxide will develop on the surface of stainless steel when it comes into contact with corrosive chemicals.
- Since it keeps steel from corroding and turning discolored, it is frequently utilized in the production of stainless steel. It is now a crucial component in steel alloying.
- Due to its tolerance to high temperatures, it is also employed in the production of nichrome, which is utilized in resistance heating components.
In Chemical Industry
One of the most common types of inorganic salts is chromium salt. It is primarily utilized in medicine, fuel, catalysts, oxidants, matches, and metal corrosion inhibitors. It is also used in electroplating, tanning, printing, and dyeing. One of the most common types of inorganic salts is chromium salt. The primary raw materials used in the chemical industry are various chromium salts that are produced from chromite.
- Surface coating is done using acidic chromate or dichromate solutions. A thin coating of chromium is placed on the metal surface using the electroplating process to accomplish this. However, a thick coating needs to be deposited in order to give wear resistant quality. Chromate conversion coating is another technique for surface coating. Chromates are used to deposit a protective layer on a variety of metals, including aluminum (Al), cadmium (Cd), zinc (Zn), silver (Ag), and magnesium (Mg).
- Because chromium (VI) salts are poisonous, they are used to protect wood from termite, fungus, and insect damage as well as deterioration. In order to stabilize leather, chromium (III) salts, particularly chromium alum or chromium (III) potassium sulfate, are employed in the tanning process.
In Refractory Materials
- Chromite, FeCr2O4 (2,190–2,270 °C), and chromium(III) oxide, Cr2O3 (2,435 °C) components for high-temperature refractory applications, like blast furnaces, cement kilns, molds for the firing of bricks, and foundry sands for the casting of metals. In these applications, the refractory materials are made from mixtures of chromite and magnesite, MgCO3.
- It may be utilized as a refractory material to line steel-making furnaces and non-ferrous metal smelting furnaces.
- Chrome bricks, chrome magnesium bricks, and other unique refractory materials are made from chromite.
In Dyes and Pigments
Chromium is also utilized in the production of pigments and colors. Chrome yellow, which is formed of lead chromate, was once widely used as a pigment. Its use fell dramatically due to environmental concerns, and it was eventually replaced with lead- and chromium-free pigments. Chrome red, chrome oxide green, and chrome green, a blend of chrome yellow and Prussian blue, are further chromium-based pigments. Chromium oxide is used to give glass a greenish tint.
Because of environmental and safety concerns, the usage of ‘chrome yellow’ has diminished. It was replaced with lead-free organic pigments or inorganic equivalents.
As a catalyst
- Several chromium compounds are utilized as catalysts in the hydrocarbon processing process.
- The ‘Phillips catalyst,’ generated by depositing chromium(VI) oxide, CrO3, on silica, SiO2, is a common catalyst for polyethylene synthesis. High-temperature catalysts for the ‘water gas shift process’ are Fe-Cr mixed oxides.
- Chromite, Cu2Cr2O5 (which frequently contains barium oxide, BaO), is an effective hydrogenation catalyst.
Health Effects of Chromium
Chromium metal is non-toxic on its own. However, when the oxidation state rises, so do the related health hazards.
- Trivalent chromium, for example, has a +3 oxidation state and is a necessary component for humans. Its absence can disrupt cellular systems such as metabolism and contribute to health concerns such as diabetes.
- However, hexavalent chromium, often known as chromium (VI), is exceedingly hazardous and produces a wide range of negative health consequences.
- Chromium levels in air and water are normally low. The quantity of chromium in drinking water is normally low as well, however polluted well water may include the deadly chromium(IV); hexavalent chromium. Because chromium(III) exists naturally in many vegetables, fruits, meats, yeasts, and cereals, consuming food containing chromium(III) is the predominant route of chromium absorption for most people.
- Different methods of food preparation and storage can change the chromium concentration in food. Chromium concentrations may grow when food is stored in steel tanks or cans.
- Hexavalent chromium can harm red blood cells. This occurs when chromium(VI) enters the circulation and initiates a series of oxidation processes. This eventually leads to in vitro hemolysis, or the rupturing of red blood cells. One serious ramification of this is that it may result in renal and liver failure.
- Chromium(VI) is hazardous to human health, particularly for those who work in the steel and textile industries. It can induce allergic responses, such as skin rash, when used as a component in leather goods.
- The National Toxicology Program (NTP) has listed chromium and most trivalent chromium compounds, including hexavalent chromium compounds; calcium chromate, chromium trioxide, lead chromate, strontium chromate, and zinc chromate as having insufficient evidence for carcinogenicity in experimental animals. The International Agency for Research on Cancer (IARC) has classified chromium metal and its trivalent derivatives as Group 3 carcinogens. (In terms of human carcinogenicity, the agent cannot be classified.)
Environmental Effects of Chromium
Chromium, like humans, is hazardous to bacteria, plants, and animals. There are various types of chromium, each with a unique effect on organisms. The two stable forms of chromium present in the environment are trivalent and hexavalent. Natural processes and human activities introduce chromium(III) and chromium(VI) into the air, water, and soil.
- Chromium compounds are frequently discovered in soil and groundwater at abandoned industrial sites due to their employment in dyes, paints, and the tanning of leather. As a result, these sites now require environmental cleanup and remediation. Hexavalent chromium-based primer paint is still often used for repairing automotive and aerospace surfaces.
- Chromium will also enter the air through coal burning, and it will enter soils through waste disposal.
- Chromium is not known to build up in fish bodies, but it can harm fish’s gills when it is present in high quantities in surface waters as a result of the discharge of metal products.
- Animals exposed to chromium may experience respiratory issues, a decreased capacity to fight sickness, birth abnormalities, infertility, and tumor development.
- Typically, plants can only absorb chromium(III). Even though this kind of chromium is necessary, when concentrations go over a certain point, adverse consequences might still happen.
Biological Importance of Chromium
- Chromium is a crucial trace element for mammals and is necessary to maintain healthy lipid and glucose metabolism.
- It enters the body through the digestive system before being sent to the tissues, where it accumulates.
- The abnormalities in metabolic processes are brought on by a chromium (III) shortage. As a result of alterations in insulin’s affinity for its receptors on cells, the fundamental response of an organism to chromium (III) deficiency is a reduced tolerance to glucose.
- Chromium (III) is found in significant amounts in nucleic acids. It affects their transcription, replication, and metabolism.
- The ion lowers the plasma level of corticosteroids and raises the immune system’s activity in an organism.
Fun Facts of Chromium
- The shiny look of chromium makes it valuable. According to research, chrome reflects around 70% of visible light. 70% of the visible light that strikes chromium will bounce off the source when it is put close to an illumination source.
- Chromium is extremely hard in addition to having anti-rusting qualities.
Watch out the video to learn an interesting fact about chromium.
References
- https://www.webelements.com/chromium/chemistry.html
- https://pilgaardelements.com/Chromium/Reactions.htm
- https://sciencestruck.com/uses-of-chromium
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- Baral, Anil; Engelken, Robert D. (2002). “Chromium-based regulations and greening in metal finishing industries in the USA”. Environmental Science & Policy
- [Biological role of chromium in humans and animals] PMID: 10609294
- Elucidating a Biological Role for Chromium at a Molecular Level: https://doi.org/10.1021/ar990073r
- Discovery, properties and applications of chromium and its compounds. http://dx.doi.org/10.1007/s40828-015-0007-z
- Theopold, Klaus H.; Kucharczyk, Robin R. (15 December 2011), “Chromium: Organometallic Chemistry”, in Scott, Robert A. (ed.), Encyclopedia of Inorganic and Bioinorganic Chemistry, John Wiley & Sons, Ltd, pp. eibc0042, doi:10.1002/9781119951438.eibc0042, ISBN978-1-119-95143-8.
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- Sreeram, K.; Ramasami, T. (2003). “Sustaining tanning process through conservation, recovery and better utilization of chromium”. Resources, Conservation and Recycling.

