The clinical application of potassium is immense in today’s world. Potassium, named after its Latin name “kalium,” which has an atomic number of 19, and the chemical symbol K. Potash, or potassium carbonate, is where potassium was first extracted (K2CO3). Since elemental potassium reacts aggressively with water, it only occurs in nature as its ion (K+), either dissolved in the ocean or coordinated in minerals.

Plants contain potassium ions, which are necessary for human health. Fertilizers, which contain various potassium salts such as potassium chloride (KCl), potassium sulfate (K2SO4), and potassium nitrate (KNO3), are the main applications for K+. While potassium bromate (KBrO3) is an oxidizing agent and is employed as a flour improver, KCl is also present in table salt. Additionally, potassium bisulfite (KHSO3) serves as a food preservative in wine and beer.
Interesting Science Videos
Biological importance of potassium ions in the human body
The phenomenon known as the action potential takes place in various excitable cells like neurons, muscle cells, and endocrine cells. It involves a brief alteration in membrane potential and holds a crucial function in intercellular communication. Within animal cells, there are two distinct types of action potentials. The first type is initiated by the activation of calcium ion (Ca2+) channels and is characterized by a longer duration compared to the second type, which is the sodium (Na+)-based action potential.
- Because of its brief duration (only 1 ms), the Na+/K+-based action potential is primarily present in nerve cells and the brain.
- Because potassium ions affect the osmotic balance between cells and interstitial fluid, they are essential for neurons to operate.
- The so-called Na+/K+-ATPase pump controls the levels of K+ both within and outside cells.
- Two K+ ions are actively carried into the cell and three Na+ ions are pumped out of it through the consumption of ATP. The end result is the establishment of the so-called resting potential, which is an electrochemical gradient across the cell membrane.
Any cell-to-cell communication occurs when modifications to the membrane initially reach a certain area of the neuron. Sodium channels found in the cell membrane will therefore open. The Na+/K+-pump creates the osmotic gradient, which allows Na+ ions to enter the cytosol and lessens the negative nature of the electrochemical gradient. The term “action potential” refers to the increase in sodium channel opening and intracellular Na+ ion flow that occurs when a threshold is met. This results in a reversal of the electrochemical gradient across the cell membrane.
- Following this, the potassium channels open and the sodium ion channels close.
- Potassium ions exit the cell because the cytosol has a higher K+ content than the extracellular fluid.
- The cell can now return to its resting membrane potential as a result. All voltage-gated K+ channels open when the membrane potential gets closer to the resting potential.
- Hyperpolarization is the term for when the membrane repolarizes beyond its resting potential.
- Reintroducing the original equilibrium of potassium and sodium ions is the final stage.
- Thus, Na+ is actively transported out of the cytosol and K+ is actively transported into it via the Na+/K+ pump.
- Thus, the original steady state is restored.
Potassium salts and their clinical application: hypokalaemia
- Ninety-five percent of K+ in the human body is contained in the cells; the remaining five percent is mostly in the blood plasma. The Na+/K+ pump carefully maintains this equilibrium, and abnormalities, such as those observed in hypo- or hyperkalaemia, can have detrimental effects.
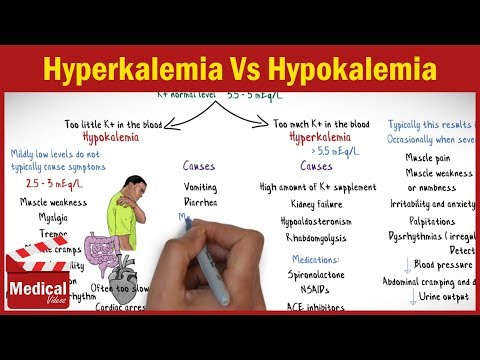
- Low levels of K+ in the patient’s blood plasma can be indicative of a potentially dangerous illness called hypokalaemia. Muscle weakness and irregularities in the electrocardiogram (ECG) are examples of symptoms.
- The most common causes of hypokalaemia are decreased K+ intake from GI disturbances such as vomiting and diarrhea or increased K+ excretion from diuresis.
- Patients receiving diuretics like thiazides and loop diuretics frequently have hypokalaemia.
- To enhance water excretion, these medication groups cause the nephrons to secrete more Na+. Unfortunately, they also cause hypokalaemia by raising K+ excretion. On the other hand, patients on thiazides or loop diuretics frequently also get potassium-sparing diuretics since they actively preserve potassium ions.
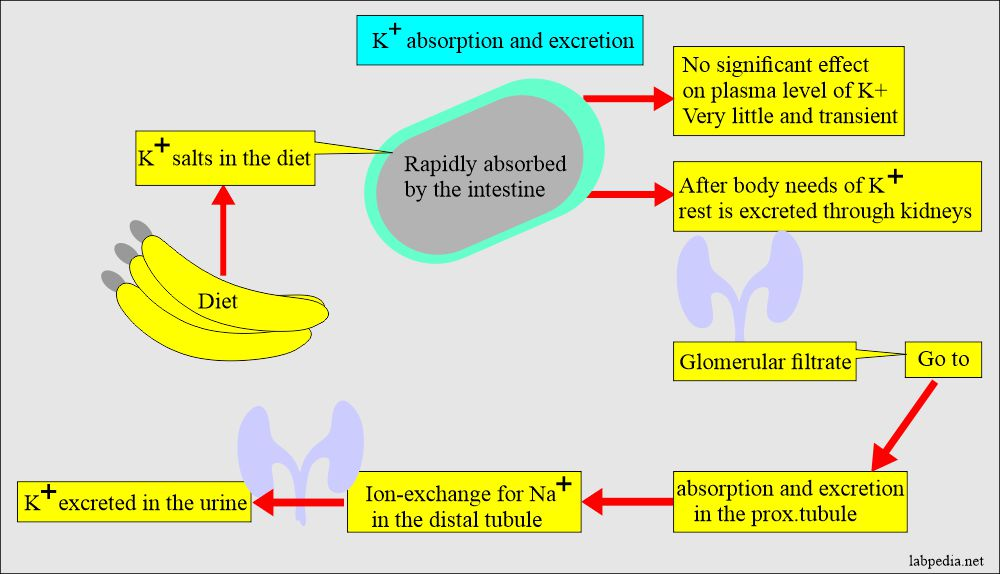
- It is the kidneys that eliminate potassium ions. Urine is produced by the kidneys filtering approximately 150–180 l of plasma every day through the glomerulus, a component of the nephron. The filtration process is succeeded by a sequence of events along the nephron that regulate plasma imbalances and control urine volume by secreting and reabsorbing a variety of ions. K+ enters the interstitial fluid through a counter-flow mechanism with Na+, primarily at the distal tubule, and is passively secreted at the proximal tubule.
- Potassium salt supplementation orally is especially important for individuals on anti-arrhythmic medications, with renal artery stenosis, severe heart failure, or substantial K+ losses from chronic diarrhea or excessive laxative use.
- When dietary changes result in a decrease in K+ intake, it may also be necessary to regulate the plasma K+ level in senior patients. However, patients with renal insufficiency must get extra attention since this may lead to a decrease in K+ excretion.
- The best way to administer potassium salts is via liquid solutions; KCl is the salt of choice.
- If the patient has elevated chloride plasma levels, or hyperchloraemia, other potassium-based salts may be administered. Potassium salts are usually dissolved in water, however they are challenging to create due to their bitter and salty flavor.
- When a patient has low blood plasma pH, or persistent acidosis, oral bicarbonate solutions, such as potassium bicarbonate, are usually administered orally. This may be caused by compromised kidney function once more.
- Potassium bicarbonate treatment for acidosis requires careful consideration because even slight variations in potassium plasma levels might have detrimental effects.
- By raising the pH of the urine, potassium citrate is used as an over-the-counter medication in the UK to relieve the discomfort associated with minor urinary tract infections. Men should not take it if they have renal pain (a risk factor for kidney stones) or if their urine contains pus or blood.
- Additionally, individuals with diabetes or high blood pressure should refrain from using potassium citrate without first talking to their general practitioner (GP). In general, patients with heart issues, renal impairment, and the elderly are urged to exercise caution.
Adverse effects and toxicity: hyperkalaemia
- There is a relatively narrow therapeutic window (3.5–5.0 mmol) for K+ in blood plasma, and hyperkalaemia—an elevated amount of K+ in plasma—in particular, can cause serious health issues.
- Potassium salts have the potential to induce nausea, vomiting, and, in severe situations, small bowel ulcers.
- When there are ECG abnormalities or a plasma potassium content greater than 6.5 mmol/l, the condition is known as acute severe hyperkalaemia. This may result in cardiac arrest, for which you should get help right away. One of the available treatment options is intravenous injections of calcium gluconate, which lessens the cardiac consequences of hyperkalaemia. The movement of potassium ions into the cells is facilitated by the intravenous infusion of soluble insulin.
- If immediate medical attention is needed, dialysis may be a viable alternative.
- Diuretics can also be administered to enhance K+ secretion in the kidneys.
- If there are no ECG abnormalities, ion-exchange resins, such as polystyrene sulfonate resins, may be used to remove excess potassium in cases of mild to severe hyperkalaemia. Potassium levels need to be closely watched and adjusted as needed, particularly in individuals with kidney disorders or end-stage renal failure. There is a chance that potassium excretion will be compromised, and an accumulation of potassium in the blood plasma could result in cardiac arrest.
- For oral use, potassium salts can also be found in pills or capsules, particularly as over-the-counter medication.
- As very high quantities of K+ are known to be hazardous to tissue cells and can injure the stomach mucosa, their formulations typically allow the potassium ions to be produced gradually. As a result, the amount of K+ in over-the-counter potassium supplements is often limited to less than 100 mg.
Video on Clinical Application of Potassium
References
- J. D. Lee, Concise inorganic chemistry, 5th ed., Chapman & Hall, London, 1996.
- https://go.drugbank.com/drugs/DB14500
- https://jamanetwork.com/journals/jama/article-abstract/326383
- https://www.webmd.com/vitamins/ai/ingredientmono-851/potassium
- https://www.pharmacy180.com/article/potassium-and-its-clinical-application