There are two categories of matter according to composition: pure substances and mixtures. A pure substance is made up of only one type of atom or molecule, whereas a mixture is made up of two or more different elements or compounds. It’s crucial for chemists to understand the composition of mixtures. We will here learn what is mixture and how to determine the composition of mixture.

Interesting Science Videos
Mixture
When two or more chemicals mix without undergoing any chemical change, the resultant substance is known as a Mixture.
Mixtures are substances that contain two or more distinct substance categories. They can be separated physically. Examples include air, different gases, a mixture of sugar and water, a solution of salt and water, and so on. Any combination’s various components do not come together chemically. As a result, the components continue to have their own qualities.
Properties of Mixtures
In contrast to chemical combinations, mixtures are composed of two or more different substances. Following is a list of the characteristics of mixtures.
- Each of the components in a mixture retains its unique features.
- The separation of components is simple.
- The proportion of each component varies.
Examples of Mixtures
- Smog: mixture of Smoke and Fog.
- Cement: mixture of Sand, Water and Gravel
- Soil: mixture of Minerals, Air, Organic materials, Water, and Living Organisms.
- Blood: mixture of Plasma, White Blood Cells, Red Blood Cells, and Platelets.
- Seawater: A mixture of various salt and water.
- Air: mixture of various gases like oxygen, carbon dioxide, nitrogen, argon, neon, etc.
- Ink: A mixture of colored dyes.
- Gunpowder: A mixture of sulfur, potassium nitrate, and carbon.
Types of Mixtures
Different types of mixtures exist depending on the nature of the components that comprise the mixtures:
Homogeneous mixture
If the same composition is present throughout the sample, the mixture is said to be homogenous. A homogenous mixture has no recognizable boundaries between its components.
Sodium chloride is dissolved in water to create a salt solution, while sugar is dissolved in water to create a sugar solution.
Heterogeneous Mixture
If a mixture has two or more components (referred to as phases) that are made up of different components, that mixture is said to be heterogeneous. It has clear lines separating its different components.
For instance, a combination of salt and sulfur, iron and sulfur, and smoke (an amalgam of air and carbon atoms).
Calculations of Composition of Mixtures
We use percentage composition in our composition calculations. The proportion of an element or compound in a substance is expressed as a percentage.
There is a gaseous combination of ammonia (NH3) and carbon dioxide (CO2) in a container. 35% of carbon dioxide is composed of this gas. If the mixture has a total mass of 76 g, how much nitrogen is there? Ammonia has a molar mass of 17.03 g/mol, while nitrogen has a molar mass of 14.01 g/mol.
First to calculate the percentage composition of ammonia
100% – 35% = 65% NH3
The mass is then calculated by multiplying the entire mass quantity by the ammonia percent composition.
65% * 76 g = 49.4 g of NH3
To calculate the molar amount of ammonia, divide the result by the molar mass. Since ammonia contains one mole (NH3) of nitrogen, the molar amounts will equal 1.
(49.4 g)/ (17.03 g mol-1) = 2.90 mole of NH3
To calculate the mass of nitrogen in the mixture, we multiply the moles of nitrogen by their molar mass.
2.90 * 14.01 = 40.06 g of Nitrogen
Composition of Gases Mixtures
The composition of a gas mixture normally measures gases by their pressure or molar amount. We employ the mole fraction rather than a percentage composition
Mole fraction (χ)
The mole fraction (χ) is the proportion of a species’ moles to the total moles of a combination.
The formula of mole fraction is:
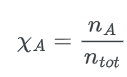
where;
- nA = number of moles
- ntot = sum of number of moles of all mixtures components
Watch out the video to learn more calculations of Composition of mixture
References
- Brown, Theodore L, H E. LeMay, Bruce E. Bursten, Catherine J. Murphy, Patrick M. Woodward, and Matthew Stoltzfus. Chemistry: The Central Science. , 2015. Print.
- Fraser, Simon. Classifying Matter According to Its Composition, LibreTexts: Chemistry, 2020.
- https://byjus.com/chemistry/mixtures/
- https://byjus.com/jee/mole-fraction/
- https://www.geeksforgeeks.org/what-is-a-mixture/
- https://library.fiveable.me/ap-chem/unit-1/mixture-composition/study-guide/dKotYK10xJPuFb5FD15l
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/composition-of-mixture/
- https://eng.libretexts.org/Bookshelves/Introductory_Engineering/Basic_Engineering_Science__Systems_Accounting_and_Modeling_Approach(Richards)/03%3A_Conservation_of_Mass/3.04%3A_Mixture_Composition

