Isomers are molecules having the same chemical formula but distinct structural configurations. Constitutional isomers and stereoisomers are two types of isomers. The structural isomers or constitutional isomers differ in the way their atoms are connected, and this phenomenon is known as constitutional isomerism. It is also known as structural isomerism. Constitutional isomers have distinct physical and chemical characteristics.
Interesting Science Videos
What are Constitutional Isomers?
Constitutional isomers are isomers that have the same chemical formula but differ in their atom arrangement, i.e. variances in bonding atom organization and bonding patterns. In other words, they have the same amount and types of atoms but are connected differently. As a result, these are sometimes referred to as structural isomers. These constitutional isomers are important in organic chemistry because of their distinct physical and chemical characteristics, as well as how they impact reactivity and behavior in chemical processes.
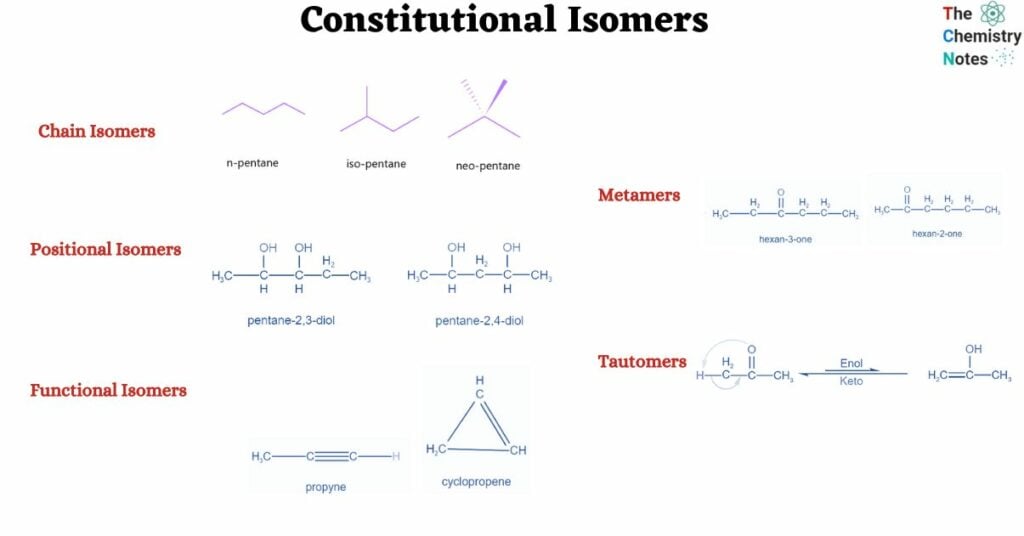
Furthermore, there exist many constitutional isomers. They are:
- Chain isomers
- Positional Isomers
- Functional isomers
- Metamers
- Tautomers
Types of Constitutional Isomers
Chain Isomers or Skeletal Isomers
Chain isomers, also known as skeletal isomers, are constitutional isomers in which the skeleton of the molecule is organized in multiple ways to produce distinct skeletal structures. This sort of isomerism is widespread in organic molecules with a lengthy carbon chain. The carbon chain in pentane, for example, may be rearranged in three distinct ways, yielding three different chain isomers:
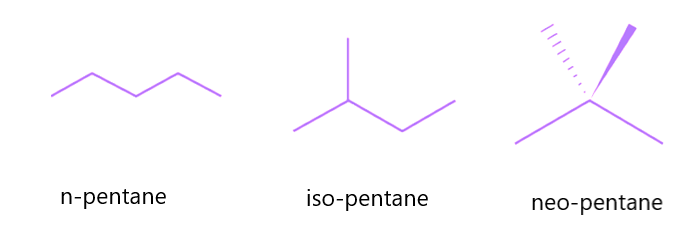
- N-pentane is the skeletal isomer of pentane with a single, five-membered carbon chain and no branching.
- Iso-pentane is the skeletal isomer of pentane, consisting of a four-membered carbon atom parent chain that branches in the second position.
- Neo-pentane is the skeletal isomer of pentane, consisting of a three-membered carbon atom parent chain that branches twice from the second position.
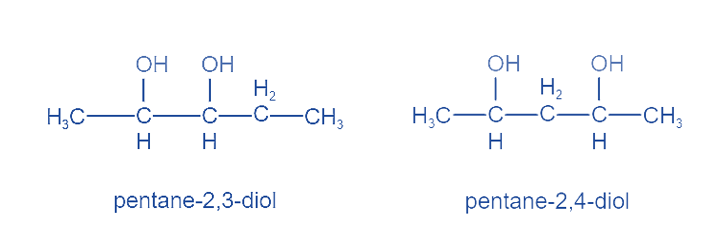
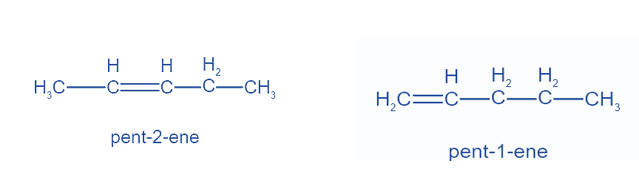
Positional Isomers or Regioisomers
Positional isomers or regioisomers differ from one another based on the location of the functional group on the molecule. For example:
Functional Isomers
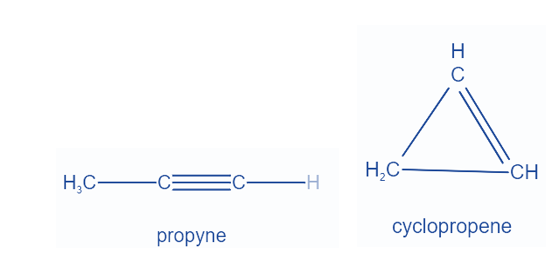
Functional isomers are constitutional isomers that have the same chemical formula but differ in how the atoms are attached to one another. 1-hexene and cyclohexane are two notable examples of functional isomerism. The former is a straight chain with one carbon-carbon triple bond, whereas the latter is cyclic with one carbon-carbon double bond.
Metamers
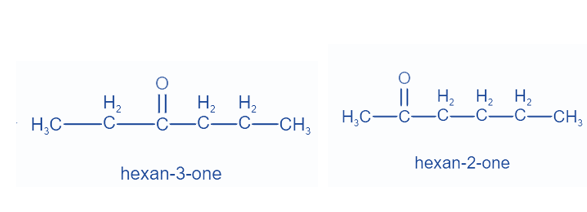
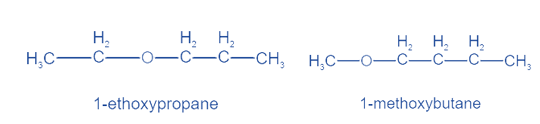
When distinct groups are connected to the polyvalent atom of the functional group, isomers are created. The formula remains the same, but the groups attached to the polyvalent atom change. Metamerism is the name given to this phenomena. Ketones and ethers display this property.
Tautomers
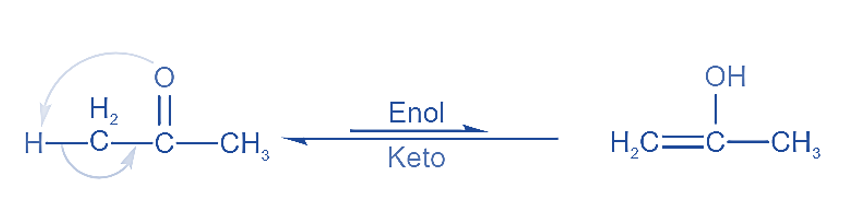
These are the constitutional isomers that interconvert quickly with one another. Tautomers cannot exist apart from one another. Tautomers exist in a state of dynamic equilibrium.
Tautomerism is the intramolecular shifting of hydrogen. Two molecular structures are easily interconvertible in this case. Tautomers are the two structures that result from H shifting. Tautomers differ only in the location of their hydrogen atoms.
The 1,3 movement of hydrogen atoms within a molecule is known as tautomerism. One of the most prevalent kinds of tautomerism is keto enol tautomerism.
Video Reference
References
- https://www.geeksforgeeks.org/isomerism/
- https://www.sciencedirect.com/topics/chemistry/constitutional-isomer
- https://byjus.com/chemistry/constitutional-isomers/
- https://www.vedantu.com/chemistry/constitutional-isomers
- https://testbook.com/chemistry/constitutional-isomers
- https://psiberg.com/constitutional-isomers/