Copernicium is a synthetic transition metal with an atomic number of 112 and is represented by the symbol ‘Cn’ in the periodic table. It is silvery in appearance and belongs to the d-block of period 7 of the periodic table. Only tiny quantities of Copernicium have been successfully synthesized, hence there isn’t much known about it based on experimental data, but some qualities can be predicted using periodic table trends.
Copernicium, an extremely radioactive element that does not occur naturally, and is produced inside a laboratory setting and decays within milliseconds after being synthesized. Victor Ninov and Sigurd Hoffmann, who were working at the GSI Helmholtz Centre for Heavy Ion Research close to Darmstadt, Germany, successfully synthesized the element 112 for the very first time in 1996. The element was named after astronomer Nicolaus Copernicus (the father of modern astronomy) and is represented by the symbol ‘Cp’.
Interesting Science Videos
History and Discovery of Copernicium
- It was produced for the first time in 1996 by Victor Ninov and Sigurd Hoffmann who worked at the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany.
- Following a lead bombardment of zinc for two weeks, they were able to produce a small number of atoms of isotope-277 of element 112, which had a half-life of 0.24 milliseconds.
- The Joint Institute for Nuclear Research (JINR) discovered isotope-284 during the decay sequence of livermorium (element-116) and isotope-285 during the decay sequence of flerovium (element-114).
- Prior to its official discovery, the copernicium was known as eka-mercury.
- After IUPAC validated the GSI team’s claim, they requested the discoverers for a name suggestion.
- On July 14, 2009, the team proposed the name Copernicium in honor of Nicolaus Copernicus. The discoverers of copernicium wanted the element’s name to honor a distinguished scientist who received little credit during his lifetime.
- On February 19, 2010, the IUPAC finally accepted the GSI team’s request to name element 112 Copernicium.
Occurrence of Copernicium
- Copernicium can be synthesized artificially. It’s a synthetic element that is extremely unstable. Its half-life is only a few seconds.
- Copernicium is a synthetic radioactive metal formed by nuclear bombardment and has only been manufactured in trace amounts.
- Copernicium is formed by blasting 208Pb with 70Zn in a heavy ion accelerator.
- Copernicium contains five isotopes having known half-lives ranging in mass from 277 to 285.
Elemental Properties of Copernicium
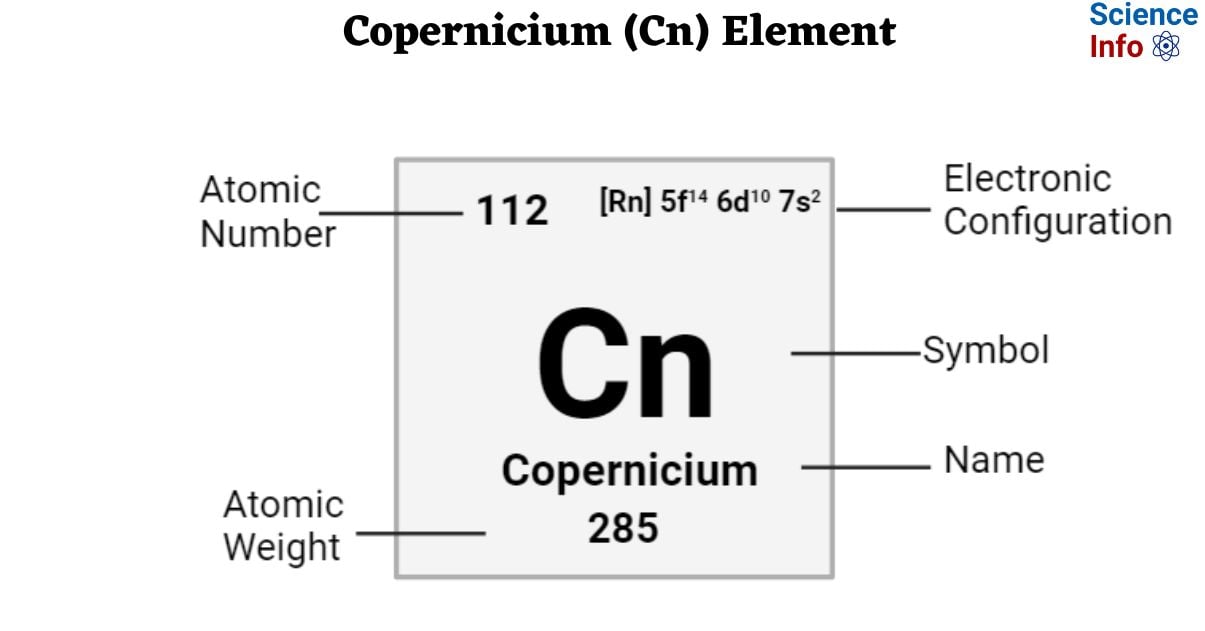
| Electronic Configuration | [Rn] 5f14 6d10 7s2 |
| Atomic Number | 112 |
| Atomic Weight | 285 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Trans-actinides, 7, d-block |
| Density | 23.21 g/cm3 (estimated) |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 18, 2 (estimated) |
| Electrons | 112 |
| Protons | 112 |
| Neutrons | 173 |
Isotopic Information of Copernicium
- Copernicium has no stable isotopes naturally, but they can be created in a laboratory setting.
- All of the Copernicium isotope are unstable and radioactive.
- All isotopes of Copernicium decay via alpha decay or spontaneous fission, but none of them undergo beta decay.
- It contains nine isotopes with known half-lives: 277Cn, 281Cn, 282Cn, 283Cn, 284Cn, 281Cn, and 286Cn.
- Isotopes are created when a heavier element decays or when two light nuclei fuse together.
- The heavier isotopes are more stable than the lighter ones.
- 285Cn is the most stable isotope, with a half-life of 29 seconds, whereas 283Cn has just 4 seconds.
- Apart for 277Cn, lighter isotopes are generated by the direct fusion of two lighter nuclei, whereas heavier isotopes are created only through the decay of heavier nuclei.
Physical Properties of Copernicium
- Copernicium’s volatility makes it difficult to perform a statistically significant study of its physical properties.
- Due to its rapid disintegration, only few properties of Copernicium have been investigated until now.
- Copernicium is a synthetic, super-heavy transactinide element.
- Copernicium’s chemistry is anticipated to resemble that of zinc, cadmium, and mercury.
- At room temperature, Copernicium is assumed to be a gas; if this turns out to be true, it will be the first element in the periodic table to exist as both a gas and a metal at the same time. (This metal can be found in a gaseous state due to its closed shell electron structure.)
- It is found in the 7th period, the 12th Group, and the d-block of the periodic table. It is regarded as a post-transition element as well as a transitional element.
- It is anticipated to be semi-conductor.
- There is no element as heavy as Cn; it is 200 times heavier than hydrogen.
- The melting point and the boiling point of the element 112 is yet to be known.
- The atomic mass of Copernicium is 285. The atomic mass of man-made trans-uranium elements is calculated using the periodic table’s longest-lived isotope. These atomic weights should be considered tentative because a new isotope with a longer half-life may be created in the future.
- Due to the relative de-stabilization of the 6d orbital and the stabilization of the 7s orbital in the copernicium atom, Cn has an electronic configuration of 5f14 6d10 7s2.
- It is expected that the crystal structure of copernicium will be close-packed hexagons.
- Copernicium isotope with the longest half-life has a half-life of 29 seconds.
Chemical Properties of Copernicium
- Copernicium is an extremely radioactive element. It’s chemical characteristics have yet to be thoroughly researched. Isotopes have short half-lives, and the molecules they contain are extremely volatile, making statistically meaningful chemical analysis difficult.
- There have been no experimental measurements of Copernicium compounds, and all known predictions are theoretical.
- Cn is projected to be a noble metal. (The chemical elements that exist in solid metal form, are exceptionally resistant to oxidation and high temperatures, have anti-corrosive properties, and do not react strongly with acids.)
- It is anticipated that the Copernicium’s chemistry would resemble that of the elements mercury, cadmium, and zinc.
- The substance exhibits high volatility and poor metallic bonding at room temperature.
- Copernicium is anticipated to form metallic bonds with silver, gold, copper, platinum, and palladium.
- It can react with gold, triggering highly volatile behavior.
- Copernicium is resistant to oxidation and necessitates unique conditions for oxidation, as opposed to the other elements of the group.
- The majority of compounds containing copernicium ions are unstable.
- Copernicium interacts with cyanide to produce the stable molecule Cn(CN)2.
- Copernicium compounds are anticipated to possess the oxidation states of +4 and +2 in aqueous solution.
Synthesis of Copernicium
- All elements with atomic numbers more than 100 can only be created through reactions in a particle accelerator, such as a cyclotron; they do not develop in a nuclear reactor.
- Copernicium-277 is produced when lead-208 is bombarded using zinc-70.
Uses of Copernicium
- Given barely any atoms of this metal have been created to date, there are currently no specific or exclusive uses of Copernicium outside of scientific research.
- Furthermore, because it is unavailable in nature, Copernicium is only employed by scientific researchers, with no recognized negative effects or uses for the metal among individuals and organizations
- A consistent scientific experiment intended to deliver an evident outcome requires a large number of atoms of the same element. However, only few atoms of Copernicium have been synthesized so far.
Health Effects of Copernicium
- Copernicium is a very unstable chemical; when created, it swiftly decomposes into other elements, therefore it has no impact on human health.
Environmental Effects of Copernicium
- Copernicium’s environmental effects are negligible due to its short half-life (just a few seconds).
Video Reference
References
- https://www.chemicool.com/elements/copernicium.html
- https://chemicalengineeringworld.com/copernicium-element-properties-and-information/
- https://periodic-table.com/copernicium/
- https://www.thoughtco.com/copernicium-or-ununbium-facts-cn-or-element-112-606611
- https://www.vedantu.com/chemistry/copernicium

