Copper is an element with the chemical symbol Cu and atomic number 29. Copper, which is classified as a transition metal, is solid at ambient temperature. Copper is a soft, malleable metal that conducts electricity and heats exceptionally well. Pure copper does not corrode; over time, it interacts with air to generate a coating of grey-green copper carbonate known as verdigris. This is seen on statues made of copper, such as the Statue of Liberty.
Pure copper is the only element that is reddish. Wires are the primary application for pure copper in electrical equipment. Copper wire wrapped around a core of iron and then electrified contributes to the creation of an electromagnet. Copper is frequently combined with other metals to create harder alloys like bronze, and brass.
Interesting Science Videos
History of Copper
Copper is one of the oldest metals discovered. There is evidence that copper was discovered during 4000 B.C., however, it may have been discovered approximately 5,000 years earlier. Almost ten years ago, UC-San Diego archeologists discovered a 70-room copper foundry at Khirbat Hamra Ifdan near the Dead Sea, which dates back to approximately 2700 B.C.; it is one of the earliest verified formal metals factories.
From prehistoric times, copper has been a necessary mineral for humankind. One of the primary “ages” or periods in human history is designated bronze, after a copper alloy. Copper and its many alloys have played a significant part in the development of several civilizations, from the ancient Egyptians and Romans to contemporary societies throughout the globe.
Copper has been used for millennia as coinage, jewelry, and adornment, but the Industrial Revolution marked the beginning of its widespread use. To meet rising demand for the versatile metal’s and its alloy families’ industrial qualities in forming, corrosion resistance, and conductivity, large-scale mining and refining processes were developed.
History of Name: Copper is derived from the Latin term ‘cuprum,’ which means ‘metal of Cyprus,’ because the island of Cyprus in the Mediterranean was an old source of mined copper. The symbol for the element Cu is also derived from cuprum.
Occurrence of Copper
Native copper can be found in many places, both as a primary mineral in basaltic lava and as a reduced form of copper compounds like sulfides, arsenides, chlorides, and carbonates. It is found in the ashes of seaweeds, several marine corals, the human liver, and numerous mollusks and arthropods. Copper performs the same function of oxygen transport in the hemocyanin of blue-blooded mollusks and crustaceans as iron does in red-blooded species’ hemoglobin. Copper, a trace element present in humans, helps stimulate the synthesis of hemoglobin.
Copper is a component of several minerals, including chalcocite, chalcopyrite, bornite, cuprite, malachite, and azurite. The largest known deposit of the mineral is a porphyry copper deposit in the Andes Mountains of Chile. At the beginning of the 21st century, Chile had become the world’s biggest copper producer. Peru, China, and the United States are additional big producers.
Copper Ores
| Azurite | Cu3(CO3)2(OH)2 |
| Bornite | Cu5FeS4 |
| Chalcocite | Cu2S |
| Chalcopyrite | CuFeS2 |
| Covellite | CuS |
| Cuprite | Cu2O |
| Malachite | Cu2(CO3)(OH)2 |
| Tenorite | CuO |
Isotopes of Copper
Copper (Cu) possesses two stable isotopes, 63Cu and 65Cu, as well as several more radioisotopes.
The great majority of radioisotopes have half-lives of minutes or less, with 67Cu having the longest half-life at 61.8 hours. Unstable copper isotopes with atomic masses less than 63 are likely to undergo β+ decay, whereas those with masses more than 65 tend to experience decay. 64Cu decays through both β+.
Cu-63 and Cu-65 are utilized to investigate copper metabolism and gastrointestinal illnesses. Cu-63 is utilized for the manufacturing of the medical radioisotope Zn-62 and can also be used for the creation of the cancer-diagnosis and therapy radioisotope Cu-64. Cu-65 has also been considered as a precursor for the creation of Cu-64 through cyclotron.
Elemental Properties of Copper
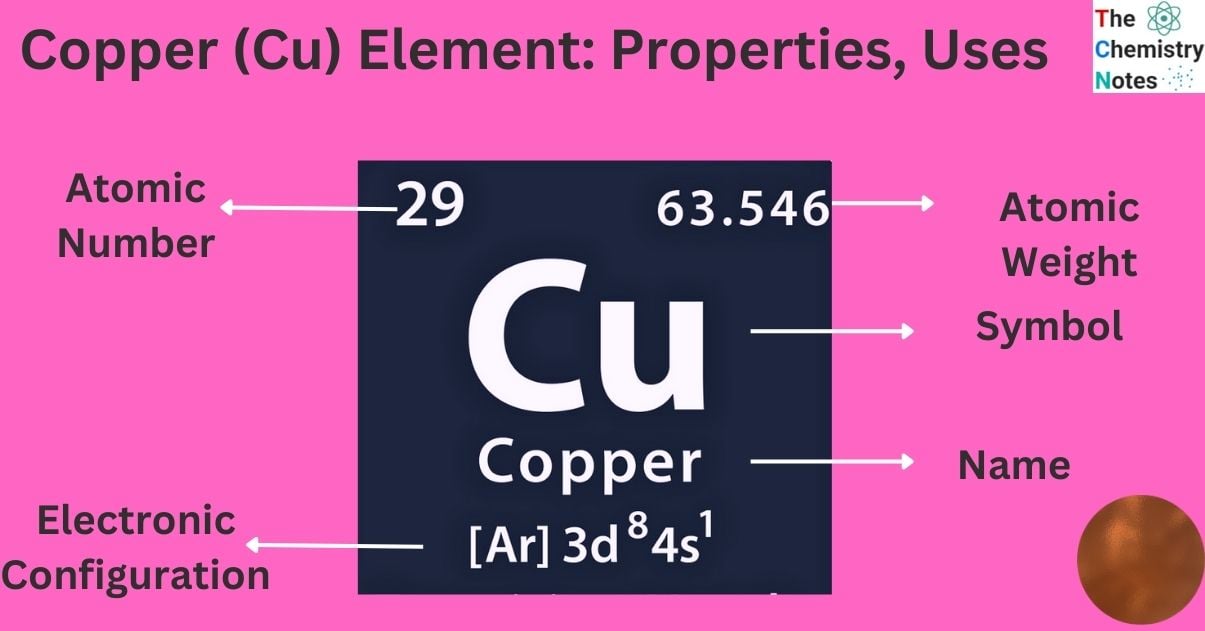
| Electronic Configuration | [Ar] 3d104s1 |
| Atomic Number | 29 |
| Atomic Weight | 63.546 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 11, 4, d-block |
| Density | 8.96 g cm-1 |
| Covalent radius | 132 ± 4 pm |
| Van der Waals radius | 140 nm |
| Electron shells | 2,8,18,1 |
| Electrons | 29 |
| Protons | 29 |
| Neutrons in most abundant isotope | 34 |
| Electronegativity (Pauling scale) | 1.90 |
Physical Properties of Copper
- Copper has a reddish color and a brilliant metallic shine. It is malleable, ductile, and an excellent electrical and thermal conductor.
- Copper is one of the most ductile metals; it is neither particularly strong nor brittle.
- Due to the production of elongated crystals with the same face-centred cubic structure as the softer annealed copper, cold treatment significantly increases the strength and hardness of the material.
- Common gases such as oxygen, nitrogen, carbon dioxide, and sulfur dioxide are soluble in molten copper and significantly alter its mechanical and electrical characteristics.
- In terms of thermal and electrical conductivity, the metal is only second to silver. Copper is composed of two stable isotopes: copper-63 (69.15 %) and copper-65 (30.85 %). (30.85 percent).
- Copper has a melting point of 1083.4 ± 0.2°C, a boiling point of 2567°C, a specific gravity of 8.96 at 20 degrees Celsius, and a valence of either 1 or 2.
| Melting Point | 1084.62°C, 1984.32°F, 1357.77 K |
| Boiling Point | 2560°C, 4640°F, 2833 K |
| Density | 8.96 g cm-1 |
| Ionization Energies | 1st: 745.5 kJ/mol 2nd: 1957.9 kJ/mol |
| Heat of Vaporization | 300.4 kJ/mol |
| Molar Heat Capacity | 24.440 J/(mol·K) |
| Electronegativity | Pauling scale: 1.90 |
Chemical Properties of Copper
The chemical characteristics of copper are diverse. These characteristics, unlike physical characteristics, can only be investigated by subjecting copper to various chemical treatments. Copper, for instance, has an extremely low reactivity. As copper reacts with other elements, it creates compounds. Copper sulfate (CuSO4), copper oxide (CuO), copper chloride (CuCl2), and copper nitrate (Cu(NO3)2) are some examples.
- Copper(II) Metal corrodes when exposed to chloride.
- Copper(II) chloride has a +2 oxidation state as a metal. It interacts with aluminum foil to produce hydrogen, Copper(I) oxide, and aluminum chloride, and is therefore a mild oxidizing agent.
- When heated, CuCl2 and NaOH react to form sodium (II) hydroxide and chlorine gas.
- Cu+1 and Cu+2 are the most prevalent ionic states of copper. When heated, Cu+2 gives out a green flame whereas Cu+1 gives off a blue one.
- Due to its position lower in the electromotive series than hydrogen, copper is insoluble in acids that continue to evolve hydrogen, but it will react with oxidizing acids like nitric and hot, concentrated sulfuric acid.
- Copper can withstand the effects of both air and water. Yet, when left exposed to the air for lengthy periods of time, a thin green protective covering (patina) composed of hydroxocarbonate, hydroxosulfate, and trace quantities of other chemicals forms.
- Copper is a somewhat noble metal because it is stable in the presence of dilute acids that are neither oxidizing or complexing in the absence of air.
- Nitric acid and sulfuric acid in the presence of oxygen will cause it to dissolve quickly. Very stable cyano complexes are formed upon dissolution, making it soluble in aqueous ammonia or potassium cyanide in the presence of oxygen.
- Cupric oxide (CuO) and cuprous oxide (Cu2O) are produced when the metal is heated to red heat with oxygen. Cu2S, or cuprous sulfide, is produced when copper is heated in the presence of sulfur.
Reaction of copper with air
Copper metal does not oxidize when exposed to air. Copper metal and oxygen combine to generate Cu2O at room temperature.
4Cu (s) + O2 (g) → 2Cu2O (s)
Reaction of copper with the halogens
By reacting with fluorine, chlorine, or bromine, copper metal produces the dihalides copper(II) fluoride, CuF2, copper(II) chloride, CuCl2, and copper(II) bromide, CuBr2.
Cu (s) + F2 (g) → CuF2 (s) [white]
Cu (s) + Cl2 (g) → CuCl2 (s) [yellow-brown]
Cu (s) + Br2 (g) → CuBr2 (s) [black]
Reaction of copper with acids
When copper metal is exposed to hot concentrated sulphuric acid, it breaks down into solutions of aquated Cu(II) ions and hydrogen gas, H2. The complex ion [Cu(OH2)6]2+ is the actual form of Cu(II) that exists in the real world.
Cu (s) + H2SO4 (aq) → Cu2+ (aq) + SO42- (aq) + H2 (g)
Copper metal also dissolves in dilute or concentrated nitric acid, HNO3.
Read Also: Fluorine(F) Element: Properties, Uses
Uses and Applications of Copper
The majority of the copper mined worldwide goes toward powering electrical devices, while the rest is used to make alloys. (It’s also useful in technology since it can be electroplated.) Some of the important applications of copper is listed below:
- Copper was the first metal to be manipulated by humans. The Bronze Age was named after this metal because of the discovery that it could be strengthened by adding tin, creating the alloy bronze.
- Cans, cooking foil, and saucepans are only a few of the many products that make use of it. Electricity lines, aircraft, and spacecraft are others. Copper is used in virtually every type of vehicle on the road or in the air today.
- Copper is also widely utilized in cutting-edge technologies, such as on-board computers, satellite tracking systems, and safety devices.
- Copper sulfate has several applications, including as a toxin for farming and as an algicide in water treatment.
- In the petroleum sector, it is used as a deodorizing agent (to mask unpleasant odors).
- Chemical assays for sugar detection employ copper compounds like Fehling’s solution.
- Copper is required in all high-efficiency electrical machinery. It is used in the construction of vats, pressure vessels, and heat exchanger components, and it is also used in the manufacture of turbine blades, bearings, and gears.
- Copper’s resistance to corrosion makes it essential for use in saltwater environments, making it a vital component of coastal power plants, oil rigs, and propellers.
- Copper sulphate is a common agricultural toxin and water purification algicide.
- While copper is commonly associated with currency, it also plays a vital role in the production of bronze.
- More than half of all copper used in the world is used to make wire, hence this property is of paramount importance. Copper is typically utilized in the generation, transmission, and distribution of electricity due to its low cost in comparison to precious metals with similar electrical conductivity. In the telecommunications industry, it is crucial in the transmission of data, especially in the context of internet access and cable television.
- The fabrication of semiconductors relies on a process called chemical vapour deposition, which entails the creation of copper films from a gaseous precursor.
- Copper is commonly plated with either gold or silver because of its popularity as a gold and silver alloy.

Health and Environmental Effects of Copper
Health Effects
- Copper is abundant in the environment and several dietary sources. Due to this, humans take in quite large amounts of copper through our diets and respiration. Copper is a vital trace element, thus it’s important for humans to take it in. Humans have evolved to tolerate relatively high copper concentrations, but ingesting too much of the metal can have serious consequences for their health.
- Copper concentrations in air are typically fairly low, therefore inhalation exposure is minimal. Nonetheless, this type of exposure is real for those who live in close proximity to smelters that convert copper ore into metal.
- Copper piping in older homes releases the metal into the water supply through corrosion, exposing the occupants to higher concentrations of the metal than the general population.
- Copper poisoning is common among workers. Copper contamination in the workplace can cause a disease similar to the flu. Oversensitivity is to blame for this condition, and it will go away after two days.
- Copper produces nasal, oral, and ocular irritation as well as stomach pain, dizziness, nausea, vomiting, and diarrhea when exposed to it over time. Deliberate copper overdoses can be fatal by damaging the liver and kidneys. It has yet to be determined if copper causes cancer.
- Wilson’s illness, caused by prolonged exposure to copper, manifests as hepatic cirrhosis, brain damage, demyelization, renal failure, and copper deposits in the cornea.
Environmental Effects
- Copper is one of the most eco-friendly metals available. Copper may be reused indefinitely since it does not deteriorate. In fact, its recycling rate is higher than that of any other engineering metal. Copper’s biostatic properties prevent bacteria from growing on its surface, making it a useful antimicrobial agent.
- Copper has a high attraction to soil’s organic matter and mineral content. Hence, it does not disperse much after emission and reaches groundwater only seldom. Copper, whether bound to sludge particles or floating freely as ions in surface water, may cover large distances.
- Since it does not degrade naturally, copper in soils can build up in plant and animal tissues. Just a select few plant species can make it on soil high in copper. That’s why you won’t find a wide variety of vegetation where copper is discarded. Copper poses a significant danger to agricultural outputs as a result of its toxic effects on plant life. Depending on the soil acidity and the availability of organic matter, copper can have a significant impact on the activities of particular farmlands. Despite this, manures containing copper continue to be used.
- As a result of its toxic effects on soil microbes and earthworms, copper can halt soil activity. This might significantly slow down the degradation of organic materials.
- Copper pollution in agricultural soils poses a health risk to animals that graze there. Sheep are especially vulnerable to copper poisoning since its effects become noticeable at low quantities.
Physiological and metabolic mechanisms have evolved in many species to control, eliminate, or detoxify copper and other toxic metals from the body. So, the notion of “bioaccumulation,” as is employed for organic compound categorization as a persistent-bioaccumulative-toxic [PBT] chemical, is incorrect since copper concentrations in tissues are not a useful predictor of prospective hazardous effects on the organism.
References
- Naturally occurring isotope abundances: Commission on Atomic Weights and Isotopic Abundances report for the International Union of Pure and Applied Chemistry in Isotopic Compositions of the Elements 1989, Pure and Applied Chemistry, 1998, 70, 217.
- https://www.metaltek.com/blog/the-history-of-copper/
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- Tables of Physical & Chemical Constants, Kaye & Laby Online, 16th edition, 1995. Version 1.0 (2005), accessed December 2014.
- J. S. Coursey, D. J. Schwab, J. J. Tsai, and R. A. Dragoset, Atomic Weights and Isotopic Compositions (version 4.1), 2015, National Institute of Standards and Technology, Gaithersburg, MD, accessed November 2016.
- https://www.thoughtco.com/copper-history-pt-i-2340112
- For further information about radioisotopes see Jonghwa Chang’s (Korea Atomic Energy Research Institute) Table of the Nuclides
- https://www.britannica.com/science/copper
- https://byjus.com/chemistry/copper/
- https://www.mindat.org/min-51758.html
- https://www.webelements.com/copper/chemistry
- https://www.lenntech.com/periodic/elements/cu.htm#:~:text=Copper%20can%20interrupt%20the%20activity,are%20damaging%20to%20their%20health.
- https://copperalliance.org/sustainable-copper/about-copper/copper-in-the-environment/
- Masses, nuclear spins, and magnetic moments: I. Mills, T. Cvitas, K. Homann, N. Kallay, and K. Kuchitsu in Quantities, Units and Symbols in Physical Chemistry, Blackwell Scientific Publications, Oxford, UK, 1988
