Defects in the crystal structure are imperfections in the crystalline solids’ regular geometrical arrangement of atoms. Temperature, pressure, impurities, and crystal formation conditions influence the size of the crystal and its perfection. As a crystal grows, various defects may appear.
Interesting Science Videos
Causes of Crystals Defects
Every large crystal has some defect in the arrangement of constituent parts. These defects result from solid deformation, high-temperature radiation striking the solid, solid deformation, and applying external stress to a crystal. The irregularities in the arrangement of constituent particles cause defects. The defects increase as the crystallization process increases.
In some cases, imperfections are intentionally introduced into crystals. The conductivity of silicon and germanium, for example, can be increased by intentionally adding arsenic or antimony impurities. This is known as “doping,” and it is used in semiconductors, which do not conduct in normal conditions. Increased conductivity is caused by the additional electrons provided by arsenic or antimony impurities (they have one more electron in their outermost shells than silicon or germanium).
Types of Crystals Defects
At absolute zero, the crystal tends to have a perfect arrangement, but as the temperature increases, the vibration of ions in their lattice site increases, and ions may jump out of the crystal lattice giving imperfection. These imperfections are also known as defects. Defects or Imperfections in crystalline solid can be divided into different groups. They are as follows.
a. Zero-dimensional defect or point defect
b. One dimensional defect or line defect
c. Two-dimensional defect or plane defect
Point defect
Ions have a very low vibrational energy at absolute zero. As a result, they are unable to leave the normal lattice sites, resulting in a perfectly ordered structure at absolute zero temperature. When the temperature rises, the thermal vibration of ions on their lattice site rises, causing them to leave their normal lattice site and form defects. A point defect is a type of defect that is only found on a specific lattice point in a crystal. As the concentration of point defects increases with increasing temperature, it is also known as the thermodynamic effect.
Point defect occurs when;
- one or more crystal atoms are missing from their respective lattice sites.
- Atom/s is moved from its corresponding lattice site to the crystal’s interstitial position.
- Foreign atoms occupy interstitial positions in the crystal lattice.
- A foreign atom replaces the original crystal atom.
Types of point defect
Point defects can be further divided into different types. They are as follows:
1. Stoichiometric defect:
Stoichiometric defects are defects in crystals that do not disrupt the stoichiometry electrical neutrality of the compound or crystal. It is also referred to as intrinsic or thermodynamic defects.
Non-ionic compounds show two types of stoichiometric defect they are:
a. Valency defect:
A vacancy defect is the point defect caused when an atom disappears from its original lattice site. It causes a void in the lattice, so the density of the substance decreases.
b. Interstitial defect:
It is a defect in which an atom or molecule occupies the intermolecular space of the crystal lattice. The atom can be of the same crystal or a different crystal/material. The density of the substance increases due to this defect. This defect causes an atomic distortion. This type of defect is generally observed in ZnO, Fe2O3, CdO, etc.
Ionic compounds also show two different types of point defect they are:
2. Frenkel defect
This defect occurs when one ion moves from its normal lattice site to an interstitial position. In general, the smaller ion (cation) moves out of its position and occupies an intermolecular space. Such defect is most commonly found in crystals with varying sizes of cations and anions. E.g., ZnS, AgCl, AgBr etc.
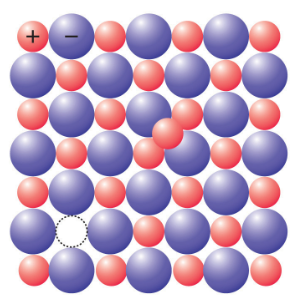
source: https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/s16-04-defects-in-crystals.html
Factors affecting Frenkel defects
The density of the Frienkel defect is determined by the following factors
a. Total number of ion pairs
b. The number of interstitial positions
c. The average amount of energy required to produce a Frenkel defect.
d. Absolute temperature
Consequences of Frenkel defects
a. The conductivity of defective salt increases as compared to non-defective salt due to the migration of ions present in the interstitial position.
b. The presence of a large number of ions in the interstitial position may distort the crystal lattice, increasing unit cell dimension.
c. As missing ions are present in the interstitial position the volume as well as mass does not change. Thus, the density of the crystal remains unaffected.
Schottky defect
It is a type of point defect in which cation and anion are lost from their normal lattice site and create a pair of vacancies or holes in the crystal. This defect, named after a German physicist, Walter H. Schottky, is common in ionic crystals with similar cation and anion sizes. Schottky defects typically appear when an ionic compound crystal is heated. Heat causes the temperature to rise, which results in an increase in the thermal vibration within the crystal. As a result, voids are created on the crystal.
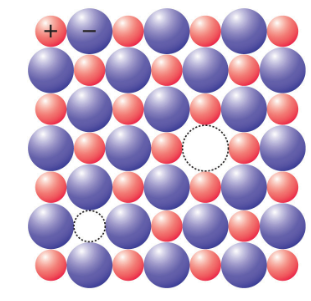
source: https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/s16-04-defects-in-crystals.html
Factors affecting Schottky defect
The density of the Schottky defect is determined by the following factors:
a. No of ion pairs per cm3
b. The average amount of energy required to produce a Frenkel defect.
c. Temperature
Consequences of Schottky defect
a. Cation and anion are removed from their normal lattice sites to the surface in the Schottky defect. Thus, as the defect increases, the volume increases while the mass remains constant. As a result, the density of defective salt decreases as compared to non-defective salt.
b. Conductivity increases when the concentration of defect increases. When electricity is passed, the nearby ion moves from its normal lattice site to the hole, thus creating a hole in the new position. During this process, the holes may migrate across the entire crystal. So, the conductivity is due to the migration of holes.
Impurity defects
There will always be impurities or foreign atoms present, and some will exist as crystalline point defects. When molten NaCl crystallizes with SrCl2, Sr2+ ions replace two Na+ ions and take the place of one Na+ ion. As a result, the lattice site of one Na+ is left vacant, resulting in an impurity defect.
There are two types of impurity point defects found in solid solutions:
1. Substitutional
One atom is replaced by an atom of a different type.
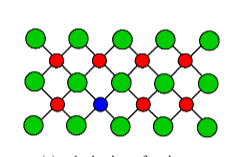
2. Interstitial
An extra atom is inserted into the lattice structure at a vacant position.
source: https://www.jobilize.com/physics4/test/point-defects-too-many-or-too-few-or-just-plain-by-openstax
Non-stoichiometric defect
Stoichiometric defects are defects in crystals that disrupt the stoichiometry of the compound or crystal. The cation/anion ratio is disturbed in this defect due to ion addition or removal. Non-Stoichiometric defects are classified into two types:
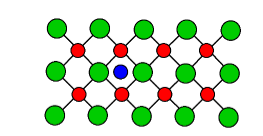
Metal excess defect
i. F-Centers (Color Centre)
In this case, the anion is absent from the normal lattice site, and the hole created by it is occupied by an electron maintaining electrical neutrality. F-centers are vacant sites occupied by electrons that are primarily responsible for color by absorbing light in the visible region.
ii. Interstitial ions and electrons
This defect arises as cation occupies an interstitial position and for electrical neutrality, an electron is included in the interstitial position. It is commonly observed in ZnO, Fe2O3, etc.
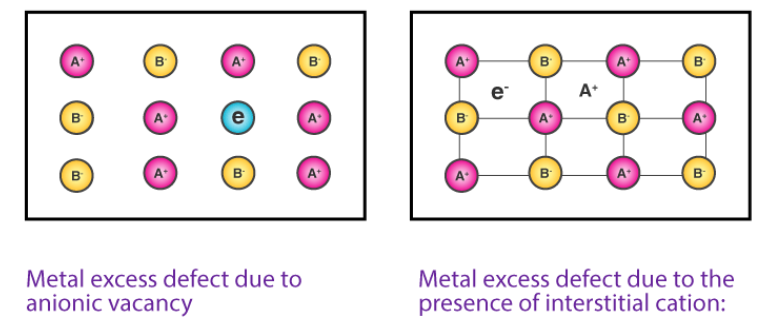
source: https://byjus.com/questions/write-a-short-note-on-metal-excess-and-metal-deficient-defect/
Metal deficiency defect
It may occur in two ways. They are:
1. Positive ions absent:
This defect occurs when one or more cations are missing from their positions, resulting in vacancies. To keep charge balance, one or more nearby cations acquire extra positive charge and are thus converted into cations with a higher oxidation state. This flaw is found in ionic crystals with positive ions that have variable oxidation states. E.g FeO, NiO.
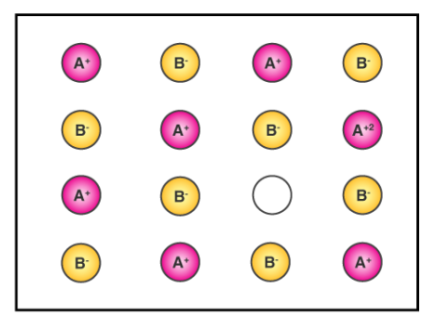
source: https://byjus.com/questions/write-a-short-note-on-metal-excess-and-metal-deficient-defect/
2. Extra interstitial negative ions
An extra negative ion is present in the interstitial position and to maintain the charge balance a nearby cation acquires an extra positive charge. However, the negative ions are usually large, it would be difficult to fit them into the interstitial position.
Consequences of metal excess defect
1. Crystal with this type of defect contains free electrons and if these migrate, they conduct electric current.
2. The crystal with metal excess defects are generally colored.
Line defect
This defect in crystal remains throughout the line called line defect. It is of two types
1. Edge dislocation:
The extra half plane of an atom is inserted into the crystal lattice in this defect. This dislocation runs perpendicular to the plane of the paper. The imperfection may run in a straight line through the crystal or it may take an irregular path. It could also be short, extending only a short distance causing a one-atomic-distance slip. The movement of dislocations, or slips, in response to applied shear stress, causes macroscopic plastic deformation.
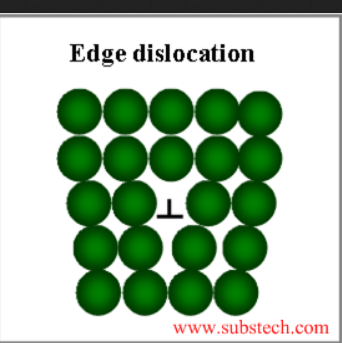
source: www.substech.com
2. Screw dislocation
A dislocation in a crystal’s lattice structure in which the atoms are arranged in a helical pattern normal to the direction of the stress. The atom in one part of the crystal is displaced relative to the rest, forming a ramp around the dislocation line. In the immediate vicinity, there is a distortion of lattice cells.
Plane defect
A planar defect is a change in the perfect crystal structure that extends across a plane. The deviation of a crystal from perfection in the plane of the crystal lattice is called plane defect.
They are the following types.
Grain boundaries:
Grain boundaries defect separates the region of different crystalline orientations within the polycrystalline solid. In polycrystalline materials, there are several small interlocking crystals or grains which are randomly oriented. The angle between the orientation of adjoining crystals is large, and the structure of grain boundaries is complex. Grains and grain boundaries contribute to a material’s properties. Grain boundaries limit dislocation lengths and motions. Smaller grains (more grain boundary surface area), therefore, strengthen a material.
Tilt boundary
A tilt boundary is formed when the grain boundaries show miss orientations of only a few degrees. As a result, a series of edge dislocations provide the tilt boundary. The elastic distortion of the crystal lattice near the boundary is associated with the interfacial energy of such a boundary.
Twist boundary
A twist boundary is formed when two crystal parts are rotated through a small angle around an axis perpendicular to the grain boundary. As a result, a series of screw dislocations provide the twisted boundary.
Stacking faults
Stacking fault occurs due to miss-matching between the pattern of the crystal lattice,
Importance of Crystals Defects
There are a lot of properties that are controlled or affected by defects.
They are as follows:
- Point defects in metals significantly reduce electricity and semi-conductivity.
- Substitution defects govern electronic conductivity in semiconductors.
- Vacancies control ionic conductivity. Vacancies control diffusion.
- Dislocation governs plastic deformation in crystalline materials.
- Defects influence the color of crystals.
- Defects strongly influence mechanical strength.
References
- https://www.vedantu.com/chemistry/point-defects-in-crystals
- https://byjus.com/chemistry/imperfections-in-solids-point-defects/
- https://biblus.us.es/bibing/proyectos/abreproy/80016/fichero/chapter+2.pdf
- https://biblus.us.es/bibing/proyectos/abreproy/80016/fichero/chapter+2.pdf
- https://www.britannica.com/science/deformation-mechanics
- A. Bahal/B.S. Bahal and G. D. Tuli, Essential of physical chemistry revised multicolor Edition, S. Chand and Co. Ltd. New Dehli 2009.
- S. Negi and S. C Anand , A textbook of Physical Chemistry, New Age International (P) Ltd. New Dehli 1999.
- https://material-properties.org/what-are-planar-defects-interfacial-defects-definition/
- https://material-properties.org/what-is-edge-dislocation-crystallographic-defects-definition/
