Curium is a synthetic chemical element with an atomic number of 96 and is represented by the symbol ‘Cm’ in the periodic table. It is silvery in appearance and belongs to the f-block of period 7 of the periodic table. Curium was identified as the third synthetic trans-uranium element in the actinide series. Similar to other elements in the actinide class, curium exhibits significant radioactivity. It is an explosive element found in almost all current nuclear weapons. American chemists Glenn T. Seaborg, Joseph W. Kennedy, and Arthur C. Wahl discovered curium in 1944. They created it by bombarding plutonium-239 with alpha particles in a 152-cm (60-inch) cyclotron in Berkeley, California.
Interesting Science Videos
History of Curium
- Glenn Seaborg, Edwin McMillan, Joseph Kennedy, and Arthur Wahl created curium for the first time in 1944. It was the third synthesized trans-uranium element from the actinide series to be identified.
- Curium-242 (half-life 162.8 days) was created by hitting plutonium-239 with alpha particles at Berkeley, California’s 60-inch cyclotron.
- During a radio show where he was invited as a guest scientist, Seaborg disclosed the discovery on November 11, 1945, following the end of World War 2.
- Werner and Perlman of the University of California, Berkeley were the first to isolate visible amounts (30Mg) of 242-Cm as hydroxide in 1947.
- W. W. Crane, J. C. Wallmann, and Burris B. Cunningham discovered in 1950 that the magnetic susceptibility of microgram samples of CmF3 was comparable to that of GdF3.
- Curium in its elemental form was first produced in 1951.
- The element has been designated after Marie and Pierre Curie, pioneers in radiation and discoverers of polonium and radium.
Occurrence of Curium
- Curium is found in trace levels in a variety of uranium minerals.
- Curium is certainly found naturally on Earth, but in extremely minute levels. Concentrated uranium deposits could generate certain atoms using the same methods that make neptunium and plutonium.
- The half-life of the longest-lived curium isotope is substantially less than the earth’s age, therefore all primordial curium has decayed by now.
- It is a synthetic element created in nuclear reactors by hitting plutonium with neutrons.
- Curium contains 15 isotopes with confirmed half-lives, ranging in mass from 238 to 252. Curium contains no naturally occurring isotopes.
Elemental Properties of Curium
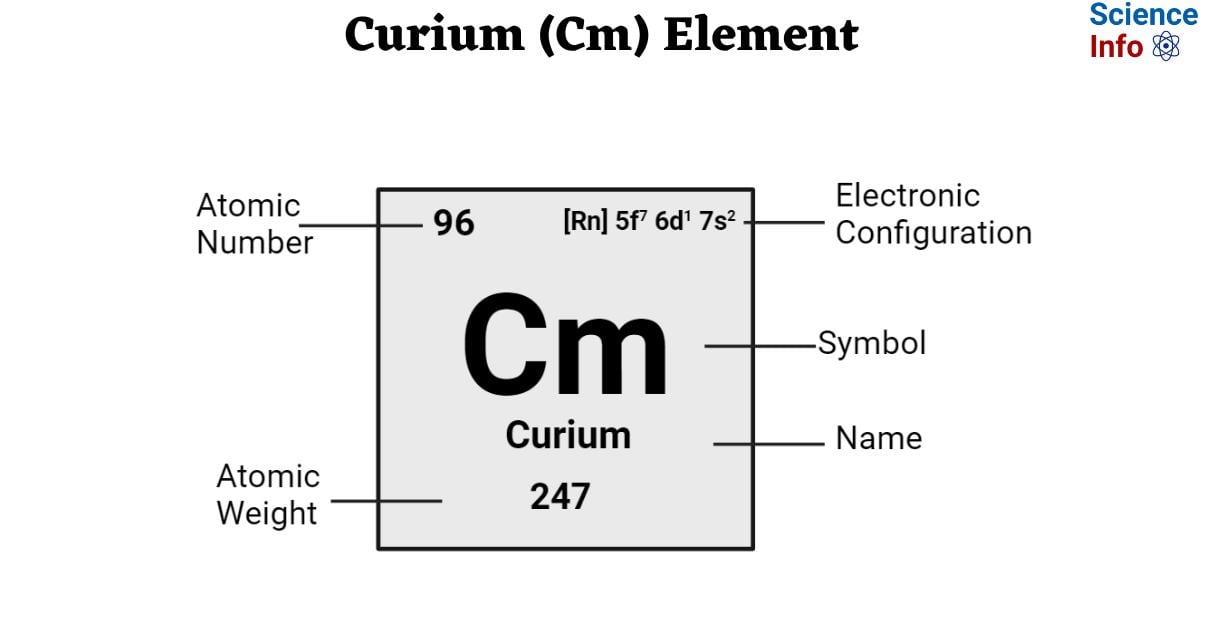
| Electronic Configuration | [Rn] 5f7 6d1 7s2 |
| Atomic Number | 96 |
| Atomic Weight | 247 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | 13.51 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 25, 9, 2 |
| Electrons | 96 |
| Protons | 96 |
| Neutrons | 151 (Varies with isotopes) |
Isotopic Information of Curium
- Curium contains 15 isotopes with known half-lives, ranging in mass from 238 to 252.
- Curium contains no naturally occurring isotopes.
- The longest-lived isotopes are 247-Cm (15.6 million years), 248-Cm (340,000 years), and 250-Cm (9,000 years).
Physical Properties of Curium
- Curium has physical properties similar to those of Gadolinium.
- The purest form of curium is a hard, brittle, radioactive silvery metal.
- Curium has a silvery-white appearance.
- The atomic mass of curium is 247.
- The melting point of curium is 1340°C. The boiling point of curium is 3383°C.
- Curium has a hexagonally symmetric structure.
- Curium deviates from Curie-Wiess para magnetism, unlike its neighbors.
- It tarnishes gradually in dry air at room temperature.
- The majority of trivalent curium compounds exhibit a slight yellow tint.
- Curium is extremely radioactive and glows red in the dark.
Chemical Properties of Curium
- Curium has chemical characteristics similar to those of Gadolinium.
- Curium ion in solution is usually always in the +3 oxidation state, which is the most stable for curium.
- A +4 oxidation state is mostly observed in a few solid phases, including CmO2 and CmF4.
- Curium ions are hard Lewis acids, therefore they form compounds with hard bases.
- Curium behaves chemically differently than actinides but similarly to lanthanides.
- Curium is known to produce oxides, halides, pnictinides, chalcogenides, and organocurium compounds.
- Curium is highly radioactive, more electropositive than aluminum, and chemically reactive.
- A few curium compounds are known as fluorides.
Compounds of Curium
Oxides
As mentioned before, the element curium has 4 states of oxidation. However, +3 oxidation compounds are most common. This metal easily interacts with oxygen, producing mostly CmO2 and CmO3 oxides. However, additional divalent oxides, such as CmO, can be found through various processes.
- Burning curium oxalate (Cm2(C2O4)3) and curium nitrate (Cm(NO3)3) in pure oxygen produces black CmO2.
(Cm2(C2O4)3) 🡪 2 CmO2 + 4 CO2 + 2 C- When CmO2 is heated in a vacuum to 600-650 degrees Celsius, it turns into whitish Cm2O3.
4 CmO2 🡪 2 Cm2O3 + O2- Another way for producing Cm2O3 is to reduce CmO2 using molecular hydrogen.
2 CmO2 + H2 🡪 Cm2O3 + H2OHalides
When fluoride ions are added to curium (III) solutions, colorless curium (III) fluoride, or CmF3, is produced. However, another halide, tetravalent curium (IV) fluoride, can only be produced by the interaction of CmF3 and molecular fluorine.
2 CmF3 + F2 🡪 2 CmF4- Curium (III) hydroxide, or Cm(OH)3, combines with anhydrous hydrogen chloride to form colorless curium (III) chloride, or CmCl3.
- Curium (III) chloride can also be converted into colorless curium (III) iodide, and light green to colorless curium (III) bromide.
These reactions occur when CmCl3 interacts with ammonium salt at temperatures ranging from 400 to 450 degrees Celsius.
Uses of Curium
- Curium is mostly utilized for scientific research.
- Because it does not occur naturally in the earth’s crust, the radioactive element has only been employed in fundamental research studies due to limited laboratory production. Furthermore, it does not react with other chemicals. However, curium-244 may be useful as a power source for radioisotope thermoelectric generators used in spacecraft.
- Curium-244 is a powerful alpha emitter that is being investigated as a potential power source in radioisotope thermoelectric generators (RTGs) for use in spacecraft and other remote applications.
- Curium-244 was utilized in the Alpha Proton X-ray Spectrometer (APXS) to determine the abundance of chemical elements in Mars’ rocks and soils.
- Curium-244 and Curium-242 are also strong alpha (Helium ion) emitters, making them suitable as alpha particle sources. Curium-244 was employed in an Alpha particle X-ray spectrometer to examine chemical element composition on space missions such as the Mars Exploration Rover and Rosetta Orbiter/Philae Lander.
Toxicity of Curium
- Curium can enter the body by food, water, or air. Curium deposits in humans in general is likely to be caused by gastrointestinal absorption of food or water. Curium is mostly eliminated from the body within a few days and just a small amount (0.05%) is absorbed into the bloodstream.
- Curium has a health risk solely when ingested. However, the odd-numbered isotopes (curium-243, curium-245, and curium-247) have a minor external risk.
Health Effects of Curium
Unless there is a local source of contaminated dust, ingestion is usually the most concerning exposure. Because curium is significantly more easily absorbed in the body when inhaled rather than swallowed, both exposure methods can be essential.
- The main health risk is bone cancers caused by the ionizing radiation emitted by curium isotopes accumulated on bone surfaces.
- Rats subjected to curium-242 and curium-244 intravenously developed skeletal malignancies, while rats exposed via inhalation developed lung and liver tumors.
Environmental Effects of Curium
- The majority of the natural curium was generated through atmospheric nuclear weapons testing, which discontinued worldwide in 1980. Accidents and other leaks from weapon manufacturing facilities have resulted in localized pollution.
- However, the amount is negligible, and its radiation contributes just a small proportion of the Earth’s background radiation. Curium oxide is the most frequent form in nature.
- Curium is often insoluble and binds very firmly to soil particles. The concentration of curium in sandy soil particles is predicted to be 4,000 times higher than in interstitial water (in pore spaces between soil particles), and it binds even more strongly to loam soil, where concentration ratios are considerably higher (18,000).
- Curium is often insoluble and binds very firmly to soil particles. The concentration of curium in sandy soil particles is predicted to be 4,000 times higher than in interstitial water (in pore spaces between soil particles), and it binds even more strongly to loam soil, where concentration ratios are considerably higher (18,000).
Video Reference
References
- https://hpschapters.org/northcarolina/NSDS/curium.pdf
- https://www.raci.org.au/common/Uploaded%20files/Periodic%20files/418.pdf
- https://www.britannica.com/science/curium-chemical-element
- https://www.chemicool.com/elements/curium.html
- https://chemicalengineeringworld.com/curium-element-properties-and-information/#google_vignette
- https://www.lenntech.com/periodic/elements/cm.htm
- https://www.vedantu.com/chemistry/curium
- https://www.theguardian.com/science/grrlscientist/2013/jul/19/chemistry
- https://www.chemistrylearner.com/curium.html

