Darmstadtium is a synthetic transition metal with an atomic number of 109 and is represented by the symbol ‘Ds’ in the periodic table. It is silvery in appearance and belongs to the d-block of period 7 of the periodic table. Darmstadtium was the fourth transactinide (super-heavy) element identified. Only tiny quantities of Darmstadtium have been successfully synthesized, hence there isn’t much known about it based on experimental data, but some qualities can be predicted using periodic table trends.
Darmstadtium, a super-heavy radioactive element that does not occur naturally, is produced inside a laboratory setting and decays within milliseconds after being synthesized. It was created artificially by Peter Armbruster and Gottfried Munzenberg in 1994 while working at the GSI Helmholtz Centre for Heavy Ion Research in Germany. The name darmstadtium honors the city where it was discovered, Darmstadt, Germany.
Interesting Science Videos
Discovery and History of Darmstadtium
- The Joint Institute for Nuclear Research (JINR) and the Gesellschaft fur Schwerionenforschung (GSI) made multiple attempts to create element-110, however, they all proved unsuccessful.
- Following that, a team led by Albert Ghiorso at the Lawrence Berkeley Laboratory (LBL) claimed to have created element-110 by bombarding bismuth with cobalt, however, their findings were never confirmed.
- Yuri Oganessian and Vladimir Utyonkov from the JINR synthesized isotope-273 of element-110 in 1994 by blasting plutonium with sulfur.
- In that particular year, Peter Armbruster and Gottfried Munzenberg at the GSI synthesized isotope-269 of element-110 by blasting lead with nickel.
- The GSI group’s evidence was regarded as more reliable than the JINR group’s, therefore they were granted permission to name element-110.
- They named it Darmstadtium. It received its name from the place where it was discovered, Darmstadt in Germany.
Occurrence of Darmstadtium
- Darmstadtium (Ds) is not found naturally in the Earth’s crust; it must be synthesized in particle accelerators. It cannot even be manufactured in a nuclear reactor.
- ‘Ds’ does not exist in nature. It is synthetically produced in limited quantities.
- Darmstadtium is a synthetic radioactive metal created through nuclear bombardment that has only been produced in trace amounts.
- Darmstadtium is obtained by blasting 208Pb with 62Ni.
- Darmstadtium has eight isotopes having known half-lives, ranging in mass from 267 to 281.
Elemental Properties of Darmstadtium
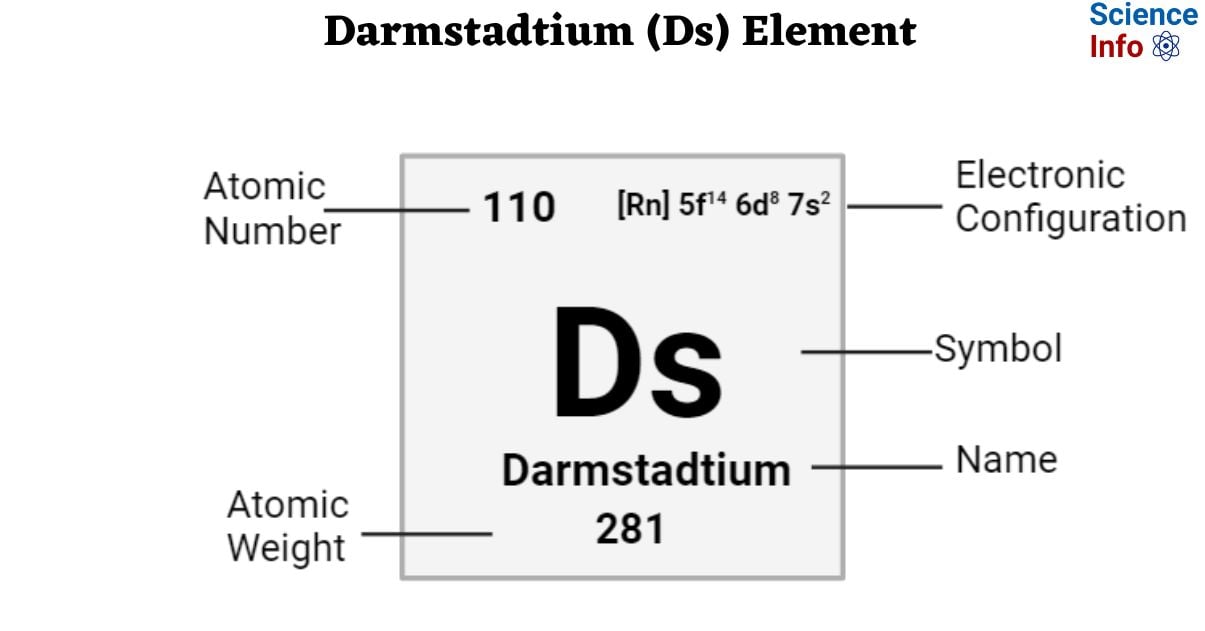
| Electronic Configuration | [Rn] 5f14 6d8 7s2 |
| Atomic Number | 110 |
| Atomic Weight | 281 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Trans-actinides, 7, d-block |
| Density | 34.80 g/cm3 (estimated) |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 17, 1 (estimated) |
| Electrons | 110 |
| Protons | 110 |
| Neutrons | 171 |
Isotopic Information of Darmstadtium
- Darmstadtium has no stable isotopes naturally, but they can be created in a laboratory setting.
- All of the Darmstadtium isotope are unstable and radioactive.
- Darmstadtium contains nine isotopes with known half-lives: 267Ds, 270Ds, 271Ds, 279Ds, 280Ds, 281Ds, and 296Ds.
- Isotopes are created when a heavier element decays or when two light nuclei fuse together.
- The heavier isotopes are more stable than the lighter ones.
- Darmstadtium-281, with a half-life of 11 seconds, is the longest-lived and most stable isotope of darmstadtium. It decays into hassium-277 by the emission of alpha particles or spontaneous fission.
- All isotopes decay via alpha decay or spontaneous fission, but none of them undergo beta decay.
- Three darmstadtium isotopes, darmstadtium-270, darmstadtium-271, and darmstadtium-281, can exist in metastable states simultaneously. Although the presence of Darmstadtium-281 has not been established.
Physical Properties of Darmstadtium
- The instability of Darmstadtium makes it difficult to conduct a statistically significant investigation of its physical properties.
- Due to its rapid disintegration, only few properties of Darmstadtium have been investigated until now.
- Darmstadtium is a synthetic, super-heavy transactinide element. It is expected to be a solid under normal conditions.
- It is projected that all elements from 104 to 111 will display fourth transition metal characteristics. This series also includes Darmstadtium, which is a platinum group metal.
- It is found in the 7th period, the 10th Group, and the d-block of the periodic table.
- It is silvery in appearance. It is projected to be silver-colored based on the difference between the ground state and the first excited state of the outer d-electrons.
- The melting point and the boiling point of the element 110 is yet to be known.
- The atomic mass of Darmstadtium is 276. The atomic mass of man-made trans-uranium elements is calculated using the periodic table’s longest-lived isotope. These atomic weights should be considered tentative because a new isotope with a longer half-life may be created in the future.
- Darmstadtium is expected to be a solid under normal conditions. It is projected to have an extremely high density of around 34.80 g/cm3.
- Under normal conditions, Darmstadtium is predicted to remain solid or crystallize in a cubic configuration with the body in its center. Aside from its lighter congeners, which crystallize in the face-centered cubic structure, it also crystallizes in the face-centered cubic symmetry.
- Apart from nuclear properties, no physical features of darmstadtium have been researched so far due to the short half-life of all of its isotope and the high cost of production.
- The longest-living darmstadtium isotope has a half-life of 11 seconds.
Chemical Properties of Darmstadtium
- Darmstadtium is a highly radioactive element. Darmstadtium’s chemical properties have yet to be fully investigated. Isotopes having relatively short half-lives, and the chemicals they contain are highly volatile, making statistically significant chemical analysis difficult.
- Darmstadtium is projected to be a highly noble metal. (The chemical elements that exist in solid metal form, are exceptionally resistant to oxidation and high temperatures, have anti-corrosive properties, and do not react strongly with acids.)
- There have been no experimental measurements of darmstadtium compounds, and all known predictions are theoretical.
- Darmstadtium is the eighth and most prevalent element of the 6d series of transition metals. They should have a similar look to platinum group metals. There are some parallels in the predictions for its ionization potentials, as well as its atomic and ionic radii. This is in comparison to its lighter homologue, platinum.
- Darmstadtium’s fundamental properties are believed to be similar to those of other group 10 elements including nickel, palladium, and platinum.
- The most stable oxidation states for darmstadtium are projected to be +6, +4, and +2.
- Darmstadtium’s chemical properties remain undetermined.
- In order to generate and cite a single molecule of element 110, billions of nickel atoms must be blasted against a lead projectile over many days, which takes many years.
Method of Synthesis
- This element was initially synthesized in 1994 at the Institute for Heavy Ion Research. During that period, the reaction was carried out by hitting accelerated nuclei of nickel-62 with lead-208 atoms in a heavy-ion accelerator.
- This reaction allowed the scientists to identify a single atom of the darmstadtium isotope: Ds- 269.
Pb-208 + Ni-62 🡪 Ds-269 + (1/0)n- In an identical test series, a larger nickel ion, Ni – 64, was utilized, and after two separate runs, the scientists identified nine Ds – 271 atoms.
Pb-208 + Ni-64 🡪 Ds-271 + (1/0)nUses of Darmstadtium
- Given barely any atoms of this metal have been created to date, there are currently no specific or exclusive uses of Darmstadtium outside of scientific research.
- Furthermore, because it is unavailable in nature, Darmstadtium is only employed by scientific researchers, with no recognized negative effects or uses for the metal among individuals and organizations
- A consistent scientific experiment intended to deliver an evident outcome requires a large number of atoms of the same element.
Health Effects of Darmstadtium
- Darmstadtium is a very unstable chemical; when created, it swiftly decomposes into other elements, therefore it does not influence human health.
Environmental Effects of Darmstadtium
- Darmstadtium’s environmental effects are negligible due to its short half-life (just a few seconds).
Video Reference
References
- https://collegedunia.com/exams/darmstadtium-chemistry-articleid-5130
- https://www.chemicool.com/elements/darmstadtium.html
- https://chemicalengineeringworld.com/darmstadtium-element-properties-and-information/
- https://www.ccdc.cam.ac.uk/elements/darmstadtium/
- https://periodic-table.com/darmstadtium/
- https://www.theguardian.com/science/grrlscientist/2013/nov/01/chemistry-chemistry
- https://thechemicalelements.com/darmstadtium/

