Dehydration synthesis, also known as a dehydration reaction, is a chemical process in which a new molecule is formed while water is removed from the result. Concentrated phosphoric acid, concentrated sulfuric acid, and alumina are some popular dehydration agents.
What is Dehydration Synthesis?
Dehydration reactions are a part of condensation reactions in which the two functional groups readily join to form a covalent bond, which is accompanied by the release of small molecules, such as water, hydrochloric acid, methanol, or acetic acid. While the aforementioned molecules are frequently seen during the large-scale commercial production of organic compounds, water is the most prevalent condensation reaction byproduct in biological systems. Because each stage involves the removal of water molecules, the reaction is known as a dehydration process, and it leads in the synthesis of a new chemical. Many biological events occur using the dehydration synthesis pathway.
There are two main prerequisites for the process to occur.
- The first criterion is the presence of a hydroxyl group in the reactant (monomer), and
- the second is the presence of another reactant containing a hydrogen atom.
Both of these monomers should be able to cleave throughout the reaction. The hydroxyl group and the hydrogen atom are released by the corresponding reactants in this reaction. These monomers combine together to form a polymer molecule, whereas the hydroxyl and hydrogen atoms combine to form a water molecule. To establish a covalent bond, electrons are shared in this process.
The production of biomolecules such as carbohydrates and proteins frequently involves dehydration synthesis. This synthesis is best recognized as a form of condensation reaction due to the nature of chemical reactions and associated products. This is because a bigger molecule is generated as a result of the aggregation or condensation of two smaller molecules, as well as the release of water.
What Happens During the Dehydration Synthesis Reaction?
- A condensation reaction takes happen during a dehydration synthesis procedure. With the removal of water molecules, the dehydration synthesis process results in the production of large molecules from two small ones.
- A dehydration reaction occurs when reactants lose one oxygen atom and two hydrogen atoms as water molecules or molecules. As a result, water is a byproduct of the dehydration reaction; other products of the dehydration process are frequently polymers formed by the joining of two reactants and the development of a double bond or a ring structure in organic molecules.
- Inorganic chemistry molecules engaged in dehydration processes, on the other hand, are often hydrates that are complexed to water molecules rather than covalently linked to them.
- Dehydration is typically reversible; they move via the dehydration and hydrolysis pathways as long as water is present in the system. Dehydrating agents are therefore substances that are often utilized to shift the reaction to the dehydration pathway.
- Dehydration synthesis mechanisms, such as the creation of di or polysaccharides from monosaccharides, are many. Another example is the creation of ether from alcohol dehydration.
Examples of Dehydration Synthesis
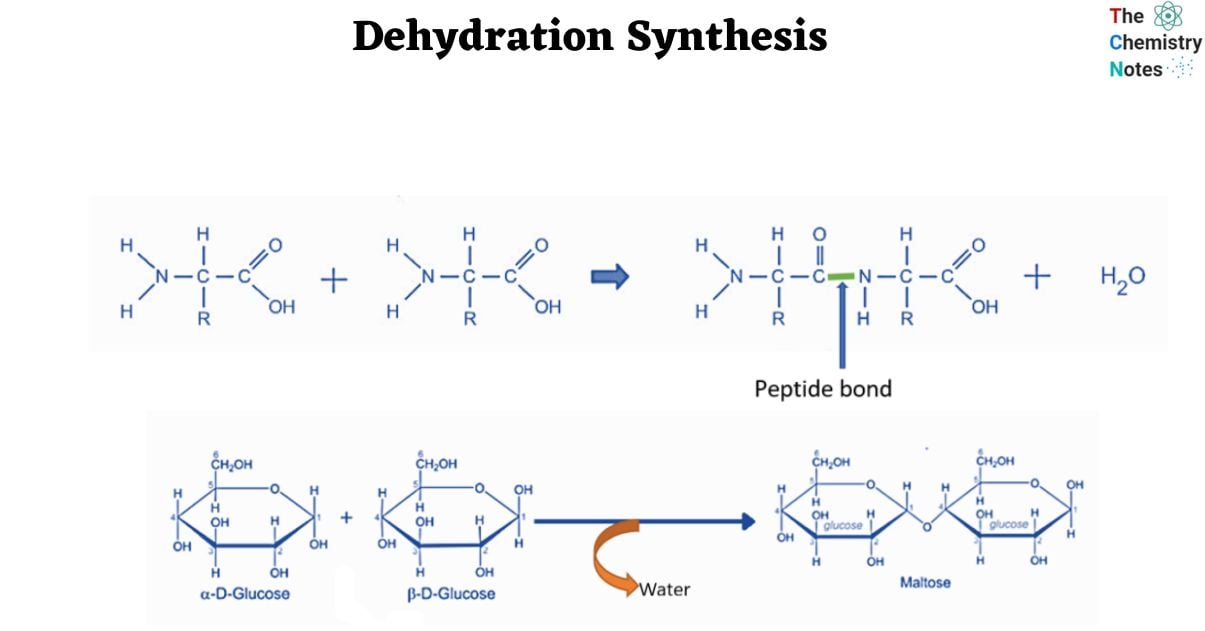
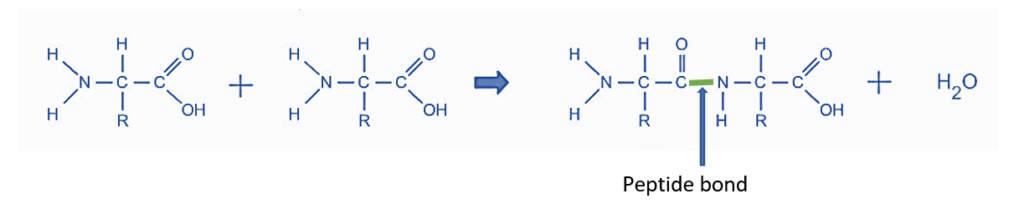
- Biochemical processes are excellent models for dehydration synthesis reactions. Peptides and polypeptides are formed when amino acids, the building blocks of proteins, polymerize. Amino acids are made up of two functional groups: amino -NH2 and carboxylic group (-COOH). With the removal of water molecules, they react to produce an amide bond (-CO-NH-).
As a result, peptide synthesis is an example of a dehydration synthesis process.
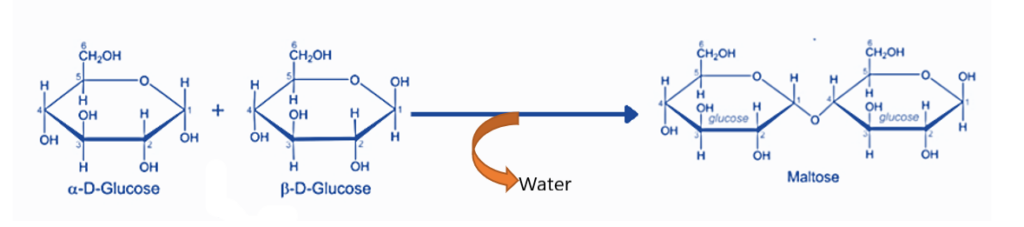
- Another example of dehydration synthesis is the formation of polysaccharides. Monosaccharides, or polysaccharide monomer units, are polyhydroxy carbonyl compounds. Monosaccharides are linked together by a glycosidic connection. This bond is produced by the interaction of two monomer units’ -OH groups with the elimination of water molecules.
Maltose synthesis is an example of a dehydration synthesis process. Two alpha-glucose units create a glycosidic bond with the removal of water molecules to generate one maltose molecule.
Dehydration Synthesis Types
There are several types of dehydration synthesis reactions based on what happens in a dehydration proces.
Biochemical reactions: This is an example of dehydration synthesis in which peptides react to produce proteins. Because amino acids are the basic building blocks of proteins. Amino acids contain two functional groups: amino (NH2) and carboxyl (COOH). Proteins are created when one amino group (NH2) of an amino acid combines with the hydroxyl group (COOH) of another amino acid, resulting in the formation of an amide bond and the release of a water molecule as a byproduct.
Condensation reactions: These are defined as the synthesis of a polymer from tiny monomers, such as the formation of polysaccharides from monosaccharides monomers. Each monomer is a polyhydroxyl carbonyl molecule that connects to the next by forming a glycosidic bond between the hydroxyl group of one monomer and the carbonyl group of another monomer, and water is released during this process. An example of a compound dehydration process is the use of glucose to manufacture maltose and glycogen.
Modification reactions: A modification reaction is one in which the dehydration process plays a major part in the modification of molecules such as phosphorylation and glycosidation. This procedure is necessary for the glycosylation or phosphorylation of proteins, nucleosides, and carbohydrates. Modification processes aid in the control of the protein kinase cascade in the human body, allowing it to carry out different functions.
Dehydration synthesis processes can be classed based on the type of catalyst utilized. Due to the fact that most dehydration reactions are reversible, a catalyst is needed to drive the reaction in only one way. Salt content, pH, temperature, and water availability in the system are examples of conditions that have altered as a result of utilizing catalysts.
Role of Dehydration Synthesis
On the Production of ATP
ATP synthase is an enzyme that converts ADP to ATP by adding a phosphate group.
Complex IV, the last complex in the respiratory chain, is also known as cytochrome c oxidase. Cytochrome c, a component of this complex, is a tiny protein that works as an electron shuttle between complex III and complex IV. Once in complex IV, these electrons concentrate in its copper cores and are transferred to oxygen via heme groups. The dehydration synthesis process to generate ATP begins here; the electrons reduce oxygen, which leads to the creation of water.
The transfer of hydrogen ions, or protons, across the mitochondrial intermembrane gap is caused by the transport of electrons along the respiratory chain. This causes an electrical potential differential across the inner mitochondrial membrane, which generates a proton motive force that powers the ATP synthase.
The addition of a phosphate group to ADP to create ATP completes the dehydration synthesis of ATP that began with the reduction of oxygen to water by complex IV.
Formation of Carbohydrate Polymers
Carbohydrates are made up of sugar molecules such as glucose, fructose, and arabinose. These sugar molecules, or monomers, can be united to produce dimers such as sucrose or lactose, polymers such as starch or cellulose, or glycosides when mixed with other molecules. This occurs as a result of a dehydration synthesis process.
Some carbohydrate polymers, such as cellulose, are not easily digested by humans and are instead fermented by our gut microbes. This fermentation process mostly produces short-chain fatty acids, which have been demonstrated to improve water and salt absorption in the colon while also supplying us with energy that would otherwise be unavailable to us. Some plant glycosides contain pharmacological qualities such as anti-inflammatory, anti-microbial, and anti-coagulant effects.
Carbohydrates are added to a molecule by the production of a glycosidic bond, which is a type of dehydration synthesis. C-, S-, N-, and O-glycosidic bonds are the four kinds of glycosidic bonds. The most prevalent kind of bond is the O-glycosidic bond. The formation of the O-glycosidic bond is a type of dehydration synthesis in which an OH group from one molecule (sugar or another molecule) reacts with another OH group from a sugar molecule to form a bond between the two molecules, resulting in the release of a water molecule.
Triglycerides Formation
Triglycerides, also known as triacylglycerols, are significant energy-storing molecules that are far more energy-dense than carbs. By esterification, three fatty acid molecules bond to a glycerol molecule. This esterification reaction is a dehydration synthesis reaction in which the fatty acids react with the alcohol groups on the glycerol molecule to create an ester bond with the release of water.
While triglycerides are necessary molecules, it has been demonstrated that an excess of triglycerides may contribute to obesity, and if this happens outside of adipose tissue, it can also lead to insulin resistance. Acyl-CoA: diacylglycerol acyltransferase 1 (DGAT1) is one of the enzymes that catalyze the dehydration synthesis of triglycerides; this enzymatic reaction is the final in human triglyceride synthesis.
Difference Between Dehydration Synthesis and Hydrolysis
| Dehydration Synthesis | Hydrolysis |
| Dehydration synthesis is a chemical process in which one or more water molecules are eliminated during a condensation reaction. | Hydrolysis, on the other hand, is a hydration reaction that reverses the dehydration reaction. Hydrolysis is the process by which large molecules’ bonds are cleaved to generate tiny molecules when water is introduced. |
| These are combination reaction. | These are decomposition reaction. |
| It forms water molecule. | Water is consumed in this type of reactions. |
| Dehydration synthesis reactants are smaller molecules than the products. | Hydrolysis reactants are more complex molecules than their products. |
| Water molecules are produced as byproducts of dehydration synthesis processes. | There are no byproducts of hydrolysis processes. |
References
- https://www.biologyonline.com/dictionary/dehydration-reaction
- https://byjus.com/chemistry/dehydration-synthesis/
- https://www.news-medical.net/life-sciences/What-is-Dehydration-Synthesis.aspx
- https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Map%3A_Raven_Biology_12th_Edition/03%3A_The_Chemical_Building_Blocks_of_Life/
- https://biologydictionary.net/dehydration-synthesis/
- https://www.biologyonline.com/dictionary/dehydration-reaction
- https://www.chemistrylearner.com/chemical-reactions/dehydration-synthesis
- https://collegedunia.com/exams/dehydration-synthesis-chemistry-articleid-5573#b
- https://pediaa.com/difference-between-dehydration-synthesis-and-hydrolysis/
- Helmenstine, Anne Marie, Ph.D. “Dehydration Reaction Definition in Chemistry.” ThoughtCo, Feb. 16, 2021, thoughtco.com/definition-of-dehydration-reaction-605001.
- https://testbook.com/chemistry/dehydration-synthesis
