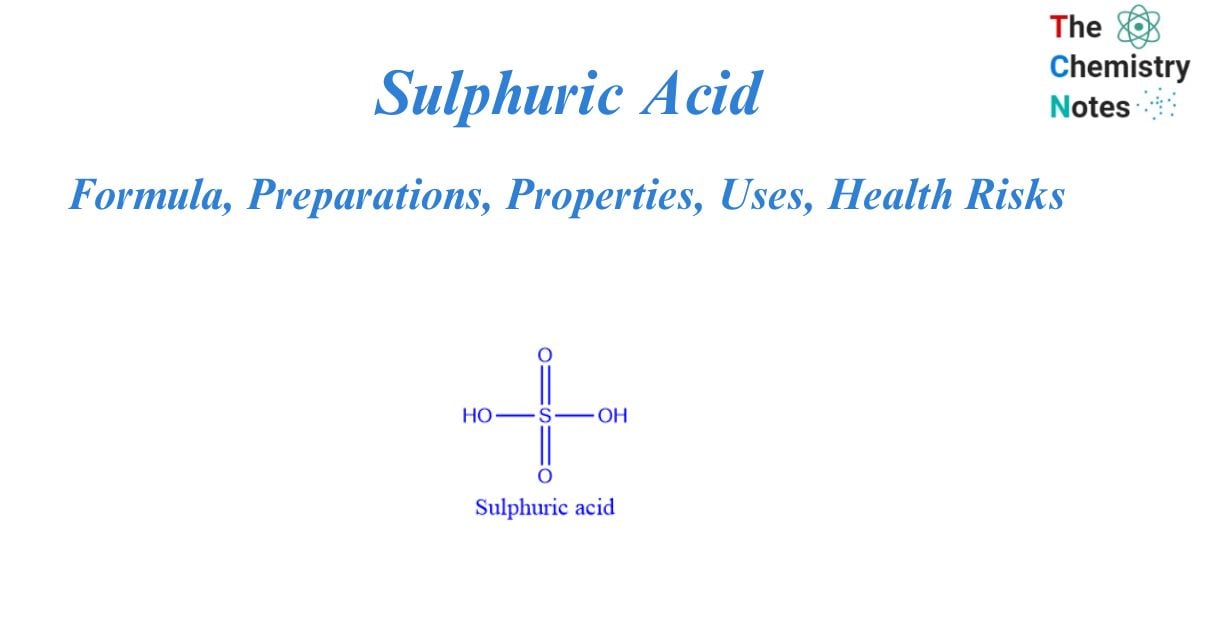
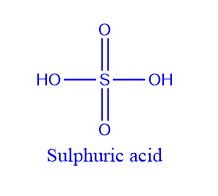
It is a well-known mineral acid that is also known as vitriol oil. It is made up of three major components: oxygen, hydrogen, and sulfur. Sulfuric acid has the chemical formula H2SO4, and it is a colorless, odorless, and viscous chemical liquid. In the eighth century, Jabir ibn Hayyan discovered sulphuric acid. Because it contains two acid protons, it is a very corrosive diprotic.
Sulphuric acid is extremely acidic. As a result, it is used to clean metals, extract impurities from oil, produce chemicals such as nitric acid and hydrochloric acid, and create dye, pharmaceuticals, detergents, and explosives, among other things.
At greater concentrations, it acts as an oxidizing and dehydrating agent. When dissolved in water, it generates heat. The acid is known by the following names:
- Sulphuric acid
- Dihydrogen sulphate
- Mattling acid
- Oil of vitriol
- Battery acid
- Dipping acid
- Electrolyte acid
Interesting Science Videos
Structure of sulphuric acid
Sulphuric acid is a sulfur oxoacid that contains two oxo and two hydroxyl groups that are covalently bonded to a central sulfur atom. The center of a tetrahedral molecule is sulfur. Sulphur is present at its maximal oxidation state, +6, in sulphuric acid. It is classified as a diprotic acid because it can dissociate into two H+ ions.
H2SO4 ⇌ 2H++SO42-
Preparation of sulphuric acid
Contact process
One mole of sulphuric acid is generated when one mole of sulfur trioxide reacts with water. In this reaction, vanadium oxide is used as a catalyst.
SO3+ H2O → H2SO4
Mechanism:
Step I: Production of sulphur dioxide
Heating sulfur or sulfide ores produces sulfur dioxide. For example, iron pyrites in excess of air.
S + O2 (Δ ) → SO2
4 FeS + 7O2 (Δ)→ 2Fe2O3+ 4SO2
Step II: formation of sulfur trioxide
2SO2 + O2 + V2O5 → SO3
Step III: Formation of oleum
The reaction between sulfur trioxide and sulphuric acid produces oleum.
SO3 + H2SO4 → H2S2O7
Step IV: Formation of sulphuric acid
oleum is diluted slowly to obtain concentrated sulphuric acid.
H2S2O7 + H2O → 2H2SO4
Lead chamber process
The lead Chamber process is one of the most used production techniques. It produces 50 to 60 B-grade acids. This technique uses wet SO2 in the presence of nitrogen oxides. As a result, it combines with the oxygen in the air to generate sulfur trioxide.
2 SO2 + O2 → 2SO3
Sulfur trioxide is then allowed to combine with water to form H2SO4. This reaction is represented by
SO3 + H2O → H2SO4
Physical properties
- H2SO4 is an oily, viscous, thick, colorless liquid.
- Sulfuric acid has a density of 1.84 g/mL, a boiling point of 337 degrees Celsius, and a melting point of 10 degrees Celsius.
- The most stable form is concentrated sulfuric acid, which is 98% water. Many more concentrations with different names are available for diverse purposes, such as battery acid (29-32%), chamber acid (62-70%), and tower acid (78-80%).
- At 298 K, it has a specific gravity of 1.84.
Chemical properties
- Sulphuric acid is a powerful dibasic acid. It is also diprotic, ionizing in two steps in aqueous solution.
- This chemical is very corrosive, reactive, and water-soluble. It has a high oxidizing power and hence functions as a potent oxidizing agent.
- It is extremely volatile. As a result, it is used in the synthesis of more volatile acids from their corresponding salts.
- Concentrated sulfuric acid is a powerful dehydrator. As a result, this chemical is employed to dry numerous wet gases that do not react with the acid.
- It also removes water from natural mixtures such as starches.
- It can oxidize both nonmetals and metals because it is a good oxidizing agent. Furthermore, it reduces to sulfur dioxide.
Reactions of sulphuric acid
Dissociation reaction
Sulfur trioxide and water are generated when pure water-free sulphuric acid is boiled.
H2SO4 → SO3 + H2O
Acidic property
A typical dibasic acid, when exposed to it, causes blue litmus to turn red.+
NaOH + H2SO4 → NaHSO4 + H2O
2NaOH + H2SO4 → Na2 SO4 + 2 H2O
Reaction with water
When H2SO4 is combined with water, an exothermic reaction occurs. The reaction generates a significant quantity of heat, to the point where the solution may even boil.
H2SO4 + H2O → H3O+ + HSO4-
As dehydrating agent
It converts glucose, sugar, and starch to carbon.
C12H22O11 + (H2SO4) → 12C + 11H2O
Precipitation reaction
When it comes into contact with aqueous solutions of barium, lead, and other salts, it produces insoluble sulfates that precipitate.
H₂SO₄ + BaCl₂ → BaSO₄ + 2HCl
Sulphonation reaction
Concentrated sulphuric acid reacts with a range of organic compounds, including benzene, toluene, and others, to generate sulphonic acids.
C6H6 + H₂SO₄ → C6H5SO3H + H2O
Reaction with metals
Concentrated sulfuric acid is a powerful oxidant. All metals except gold, iridium, platinum, rhodium, and tantalum can be oxidized by it.
- Hot and concentrated sulfuric acid reacts with metals such as aluminum, reducing to H2S, S, and SO2.
8 Al + 15 H₂SO₄ (concentrated) → 4 Al₂ (SO₄)₃ + 12 H₂O + 3 H₂S
- When copper combines with hot, concentrated sulfuric acid, the following reaction occurs:
Cu+ 2 H2SO4 → CuSO4 + SO2 +2 H2O
- A common reaction is the action of sulfuric acid on zinc to produce hydrogen. When zinc granules are added to dilute sulfuric acid, hydrogen gas is formed while the metal dissolves.
Zn + H₂SO₄ → ZnSO₄ + H₂
Uses of sulphuric acid
- 93% pure sulfuric acid is employed in the refining of petroleum products.
- Sulfuric acid is used to pickle steel before tinning and galvanizing.
- Sulfuric acid is used in metallurgy to remove (float) copper, zinc, and lead from their ores. It is also used to leach ores and waste tailings for metal recovery.
- Sulfuric acid is a laboratory reagent used in acid-base titrations, water analysis, polymer testing, redox titration, and other applications.
- Under typical conditions, alcohols and aldehydes can be oxidized to carboxylic acids by employing dilute sulfuric acid.
- It is used in the production of superphosphate, ammonium sulfate, and other chemicals.
- It is used to make metal sulfates such as zinc, copper, aluminum, magnesium, and so on.
- It is used in the production of dyes, paints, explosives, and detergents. It is also utilized in electrochemical cells and batteries as an electrolyte.
- It is used to purify substances such as benzene, paraffin oil, petroleum, and others.
- This is commonly used to make nitroglycerin, pyroxylin, nitrobenzene, picric acid, and nitro ethers.
- They are mostly utilized in the distillation of fatty acids.
- H₂SO₄ is mostly utilized in the “wet technique” of producing phosphoric acid.
- It is employed in the manufacturing of chemotherapeutic drugs, as well as to damage the DNA of cancerous cells. It can be found in ointments used to treat several skin disorders. Debacterol, a topical ointment intended to treat cancer sores, has it as an active ingredient.
- It is used in the production of fertilizers.
Health risks associated with exposure to sulphuric acid
- Skin contact might result in necrosis.
- It irritates the upper respiratory tract greatly.
- Sulfuric acid can cause severe lung damage and emergencies.
- It irritates the eyes and can potentially lead to blindness.
- It can induce fluid buildup in the lungs. Pulmonary edema is the medical term for this illness.
- Headaches, vomiting, and nausea can all be caused by sulfuric acid exposure.
References
- https://www.toppr.com/guides/chemistry/the-p-block-elements/sulphuric-acid/
- https://byjus.com/chemistry/sulfuric-acid/#:~:text=Sulfuric%20Acid%20is%20a%20mineral,odourless%20and%20has%20no%20colour.
- https://www.aakash.ac.in/important-concepts/chemistry/uses-of-sulfuric-acid
- https://unacademy.com/content/jee/study-material/chemistry/reaction-with-sulphuric-acid/
- https://www.geeksforgeeks.org/sulfuric-acid/
