Diazotization reaction is the reaction that produces diazonium ions from aromatic amines. The process of creating a diazonium salt or diazonium compound is known as diazotization. An aromatic amine combines with a reagent having a nitrosyl cation (NO) or a reagent capable of generating the appropriate aryldiazonium salt in this organic process. Diazotization is the chemical process that converts a primary aromatic amine into its equivalent diazonium salt.
In 1858, the German industrial chemist Peter Griess was the first to report such a reaction. He went on to find many more diazonium salt reactions.
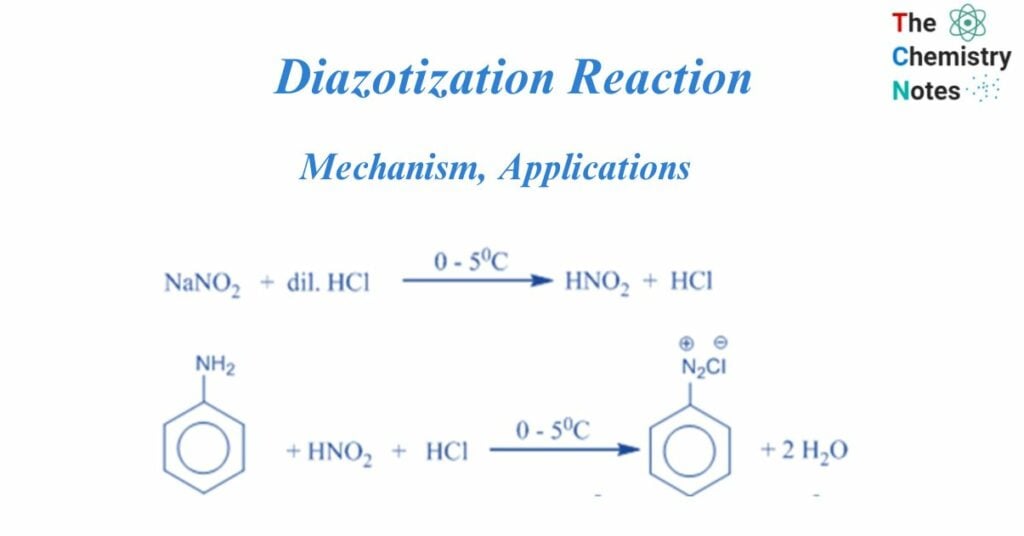
These diazonium salts are often created by reacting an aromatic amine with nitrous acid in the presence of another acid. For example, in the process below, aniline interacts with sodium nitrite and hydrochloric acid to generate nitrous acid. At temperatures ranging from 0 to 5°C, the nitrous acid interacts with aniline to create diazonium ions. Tetrazotization refers to the diazotization of bifunctional aromatic amines (for example, p-phenylenediamine or benzidine).
Interesting Science Videos
What is the Diazotization reaction?
Aromatic amines react with nitric acid and mineral acids to make diazonium salts, and water is produced as a byproduct. This is referred to as the diazotization reaction.
Mechanism of Diazotization reaction
When primary amine is treated with nitrous acid, the diazonium salt is formed, which is a compound of the type Ar/R-NN+ X–, where X- is an anion such as chloride, bromide, sulfate, and so on. However, because nitrous acid is unstable, it is produced in situ by treating sodium nitrite with a strong acid such as HCl or H2SO4. Diazotization is the process of converting primary amine to its diazonium salt.
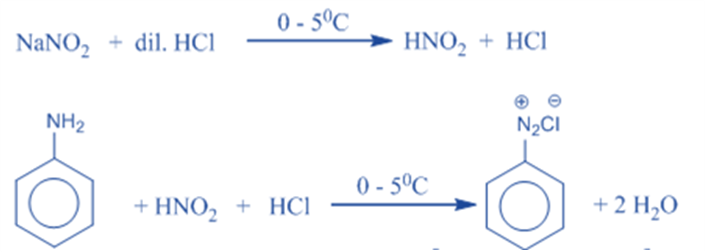
Generation of nitrosonium ion
The production method of diazonium salts involves the addition of NO, followed by a sequence of acid-base events that convert the oxygen into water and establish a triple bond between the two nitrogens. Afterward, the water is released as a leaving group.
The unstable nitrous acid must first be produced by treating sodium nitrite with aqueous HCl. Nitrous acid is formed by protonating a nitrite ion with HCl. The nitrous acid is then protonated to produce an oxonium ion with a suitable leaving group (water). Finally, the removal of the leaving group results in the formation of nitrosonium ion or nitrosyl cation.
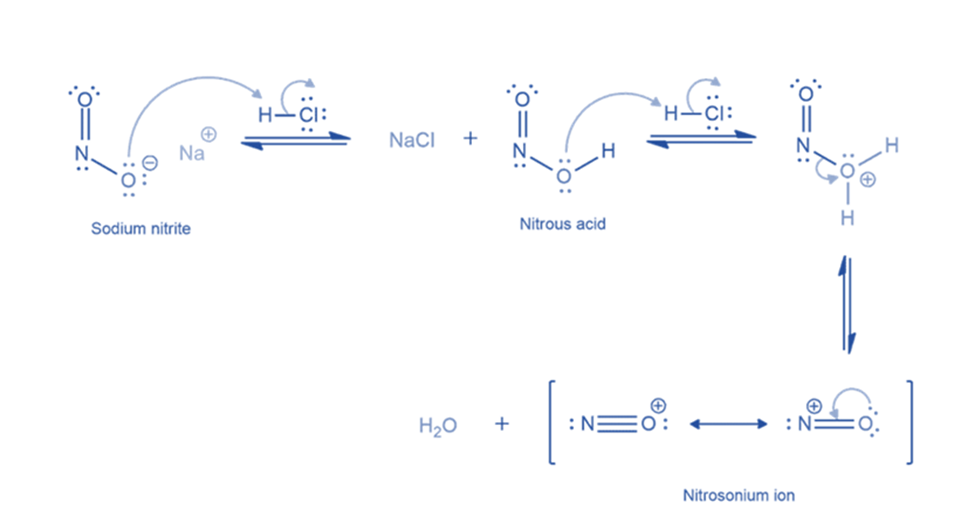
Formation of benzene diazonium ion
The nitrosonium ion is electrophilic, and amines can attack it to create an N-nitrosonium salt. When water deprotonates this nitrosammonium ion, a stable intermediate (N-nitrosamine) is generated. The oxygen atom of N-nitrosamine is then protonated, and water removes a proton from the nitrogen atom to form a double bond between the nitrogen atoms. The oxygen atom is protonated once more, resulting in a good leaving group (water). The departure of water results in the formation of a diazonium ion.
Applications of Diazotization Reaction
- Diazotization reactions are commonly used in the synthesis of azo dyes. Azo dyes are a type of organic compound that is widely utilized in the textile, printing, and coloring industries.
- Various colored azo compounds can be synthesized by diazotizing an aromatic amine and coupling it with a suitable coupling agent.
- Aryl halides, such as aryl chlorides or aryl bromides, can be created from aromatic amines through the Diazotization reaction.5
- In the production of medicinal substances, diazotization processes are used. The resulting diazonium salts can be reacted with a variety of nucleophiles, including phenols, amines, and thiols, to form pharmaceutical intermediates or active pharmaceutical ingredients (APIs).
- Diazonium compounds are common reagents in the synthesis of organic molecules.
- Diazonium salts have the potential to be employed in nanotechnology. They help single-walled nanotubes to be functionalized effectively.
- Aromatic fluorides can also be produced through diazotization processes.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
