Nuclear reaction
Nuclear reactions are the process in which two atomic nuclei or one atomic nucleus and a subatomic particle (such as a proton or neutron or high energy electron) collide to form one or more nuclides different from parent nuclei.
e.g., 63Li + 11H → 32He + 42He
Chemical reaction
A chemical reaction is a process in which one or more chemical elements or compounds react to give different products. In this reaction, the constituent atoms of the reactants are rearranged to produce different substances as products.
e.g., CH4 (g) + 2 O2 (g) → CO2 (g) + 2H2O (l)
Interesting Science Videos
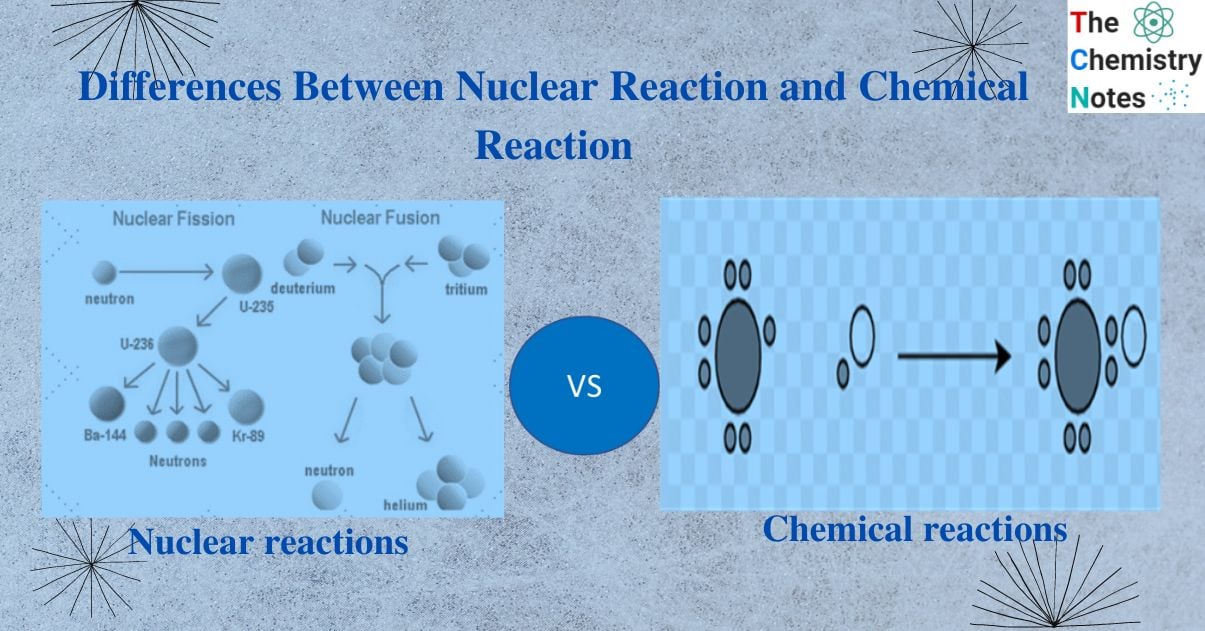
Differences between nuclear and chemical reactions
Nuclear reactions are completely different from the chemical reactions. Some important points area as follows:
| S.N | Nuclear reaction | Chemical reaction |
| 1. | Two atomic nuclei collide to form one or more nuclei different from parent nuclei. | One or more chemical elements or compounds react to give different products. |
| 2. | Changes occur in the nuclei. | Changes occur in extra nuclear region. |
| 3. | The nucleons are arranged, and one nucleus is transformed into another. | Atoms are arranged by the breaking and forming of bonds. |
| 4. | External factors like temperature, pressure, and other environmental conditions do not affect the rate of nuclear reactions. | External factors like temperature, pressure and other environmental conditions affect the rate of chemical reactions. |
| 5. | Reaction is rare. | Reactions are common. |
| 6. | The reaction is accompanied by the absorption or release of a tremendous amount of energy. | The reaction is accompanied by the absorption or release of a small amount of energy. |
| 7. | It involves the emission of lighter particles like α, β, γ and so on. | It involves the loss or gain of electron or overlapping of atomic orbitals. |
| 8. | These reactions can not be controlled easily. | These reaction can be controlled easily. |
| 9. | These reactions follow the law of conservation of mass. | These reactions do not follow the law of conservation of mass. |
| 9. | Eg., 23592U + 1on → 14456Ba + 9036Kr + 2 10n | E.g., Cu + 2 H2SO4 → CuSO4 + SO2 + 2 H2O |
References
- R.D Madab, B.S Bisht 2018. ISC chemistry.: S .Chand publications.
- S, P. (2011, October 11). Difference Between Nuclear reaction and Chemical reaction. Difference Between Similar Terms and Objects.
- ht tp://websites.umich.edu/~ners311/CourseLibrary/bookchapter17.pdf
- http://sarsunacollege.ac.in/WebPages/Downloads/ELearning/Science/Physics/UG%204th%20Sem/fission_and_fusion_teacher_notes.pdf
- https://www.britannica.com/science/chemical-reaction
- https://socratic.org/questions/what-are-six-differences-between-nuclear-reaction-and-chemical-reaction
