A dye is a chromatic compound typically employed in a solvent to create a substance that has the ability to adhere to a textile. The dye must possess exceptional ‘fastness’ or chemical stability to make sure that the hue remains impermeable to the effects of cleansing agents and water, as well as resistant to the detrimental impact of solar radiation, among other factors.
A dye is a pigmented substance that possesses a strong attraction for the substrate onto which it is being administered. It is a highly electrifying and redolent organic compound. The pigment is commonly administered in an aqueous solution and may necessitate a mordant to enhance the permanence of the pigment on the fiber.
The process of dyeing typically takes place within a specialized solution comprised of dyes and specific chemical components. Following the process of dyeing, the dye molecules establish an unbroken chemical linkage with the fiber molecules. A naturally occurring or artificially derived substance employed to imbue pigmentation or alter the chromaticity of an object.
Dyes are the pigmentation agents that imbue the articles of our daily lives with vibrant hues. Dyes are ubiquitously employed, spanning from plastic playthings for juveniles to the textiles adorning your personage, from comestibles to timber; scarcely any sector exists wherein dyes are not commercially utilized.
Interesting Science Videos
History of Dyes
- The discovery of dyed flax fibers has been documented in a prehistoric cave situated within the Republic of Georgia, with the cave dating back to approximately 36,000 years before the present.
- Archaeological findings indicate that the practice of dyeing has been extensively conducted for a duration exceeding 5,000 years, with notable prevalence observed in regions such as India and Phoenicia.
- The earliest documented evidence of the utilization of dyestuffs can be traced back to the year 2600 BC in China. The historical practice of preparing and utilizing dyestuffs is considered to be one of the earliest manifestations of human activity.
- Archaeological excavations yielded evidence in the form of ancient fabrics that were unearthed at various archaeological sites. References to it are additionally found in the Bible and other literary works from classical antiquity.
- Significant advancements in the field of dyes occurred in 1856 when an adolescent conducting experiments in a rudimentary home laboratory made a pivotal discovery that served as a catalyst for the subsequent development of the contemporary chemical industry.
- William Henry Perkin, an English chemist at the age of eighteen, made an accidental discovery of the first synthetic dye while conducting research on synthetic quinine as a potential treatment for malaria. The researcher discovered that the oxidation of aniline has the ability to impart color to silk.
- A reddish-purple dye was synthesized from a coal tar derivative. The dye exhibited poor stability when exposed to sunlight or water, resulting in significant fading to its current shade known as mauve, which is a light purple color.
Different Types of Dyes
Dyes are categorized based on their chemical composition, the specific fibers to which they can be effectively applied, their hue, or the manner in which they are applied. Dye molecules have a tendency to bind to the surface of the fiber, penetrate the fiber, or interact with the molecular constituents of the fiber. Dyes elicit distinct responses from individual fibers. Diverse responses are observed among various fiber modifications when subjected to identical dyeing processes. In the realm of dye classification, it is imperative to acknowledge that diverse hues exhibit varying degrees of colorfastness.
Natural/Organic Dyes
- Most of the time, the word “natural dye” refers to substances that come from plants. However, cochineal, kermes, and lac, which come from insects, are also included in this category.
- Natural dyes are made up of many different parts that come from plants, animals, or rocks. A wide range of plants, such as herbs, shrubs, and trees, as well as insects, animals, microbes, and minerals, have been carefully cataloged and found to have the potential to produce colors through extraction processes.
- Natural dyes can be used to make many different colors, such as red, yellow, brown, blue, black, green, and orange.
Sources of Natural Dyes
Natural dyes comprise a wide range of chemicals obtained from natural sources. The dyes can be classified into plant, animal, mineral, and microbial dyes based on their distinct sources of origin. It is important to acknowledge that plants primarily function as the principal sources of natural dyes. The sustainability of natural dyes is derived from their intrinsic properties of being renewable and biodegradable. The categorization of natural dyes is determined by their origins and can be classified into the subsequent groups:
- Plants
Various parts of plants, such as roots, leaves, fruits, flowers, and bark, have been identified as viable sources for obtaining natural colors. Various hues can be derived from distinct components, for instance, the pods of the Sappan-wood trees yield a red pigment, the barks produce a brown shade, and the roots offer a yellow dye. Various by-products derived from plants can also be utilized for the purpose of dye formation.
- Animals
Dyes with multiple hues can be derived from the desiccated remains of insects, such as Lac, Cochineal, and Kermes, serving as prime examples. Cochineal, an exquisite crimson dye, is derived from the insects that inhabit Cactus plants. The production of carmine and Tyrian purple dye involves the utilization of cochineal and shellfish (specifically Murex), respectively.
- Microbes
Natural colorants have the potential to be obtained from quickly growing fungi, bacteria, and algae, showing great possibilities for achieving commercial uniformity. In the process of dye extraction, Chitosan, Serratia spp. (a type of bacteria), Trichoderma virens (a type of fungus), and Alternaria alternate (another type of fungus) were used. The production of the natural red pigment is attributed to Monascus anka and Echinodontium tinctorium fungi. Phycocyanin, a chromoprotein exhibiting a blue hue, is obtained from the algal species Spirulina plarensis.
- Minerals
Mineral dyes encompass a range of pigments, namely iron buff, iron black, manganese bistre, chrome yellow, and Prussian blue.
Properties of Natural Dyes
Natural dyes, being of organic nature, are obtained through the process of derivation or extraction from resources that are inherently present in the natural environment. The utilization of natural dyes is predominantly characterized by their environmentally friendly nature, biodegradability, reduced toxicity, and diminished allergenic potential when compared with their synthetic dye counterparts. The properties of natural dyes are listed below:
- The utilization of organic dyes presents a safer alternative to synthetic dyes, as they lack the presence of potentially hazardous chemicals. Consequently, the likelihood of inducing skin allergies is significantly diminished. The majority of plant-derived dyes are considered to be harmless for application in textile applications.
- Natural dyes possess biodegradable properties, thereby mitigating any adverse impact on the water ecosystem upon their discharge into aquatic environments.
- Fabrics that are colored using organic dyes exhibit increased fragility and necessitate cautious handling, thereby precluding direct exposure to sunlight during the drying process.
- Natural dyes often demonstrate a pronounced propensity for significant fading. As a result, fixatives are utilized to augment the affinity of these dyes toward textile materials. The fixing agents utilized in this context can manifest as various substances, including starch, seaweed, Alum (a hydrated double sulfate salt), table salt, or vinegar.
Synthetic Dyes
- Synthetic dyes include a wide range of dyes generated from organic or inorganic chemicals. Synthetic dyes are created by combining a wide range of chemical components.
- The most common method for acquiring dyes is based on their cost-effectiveness, optical properties pertaining to color, and durability in terms of fastness and mordancy.
- The use of synthetic colors gradually overtook the traditional use of natural dyes. The synthetic goods were more cost-effective, providing a varied range of new color possibilities while simultaneously enhancing the intrinsic properties of the dyed fabrics.
- Dyes are currently categorized based on their use in the dying process.
Types of Synthetic Dyes
Advancements in the field of synthetic organic chemistry have facilitated the emergence and subsequent production of synthetic dyes. Various types of dyes are employed for the purpose of coloring a wide range of fabrics, including cotton, wool, silk, rayon, acetate, polyester, nylon, and acrylic, among others. The colors allocated to each specific type of fabric are enumerated in the following list:
Water Soluble Dyes
- Acid Dyes
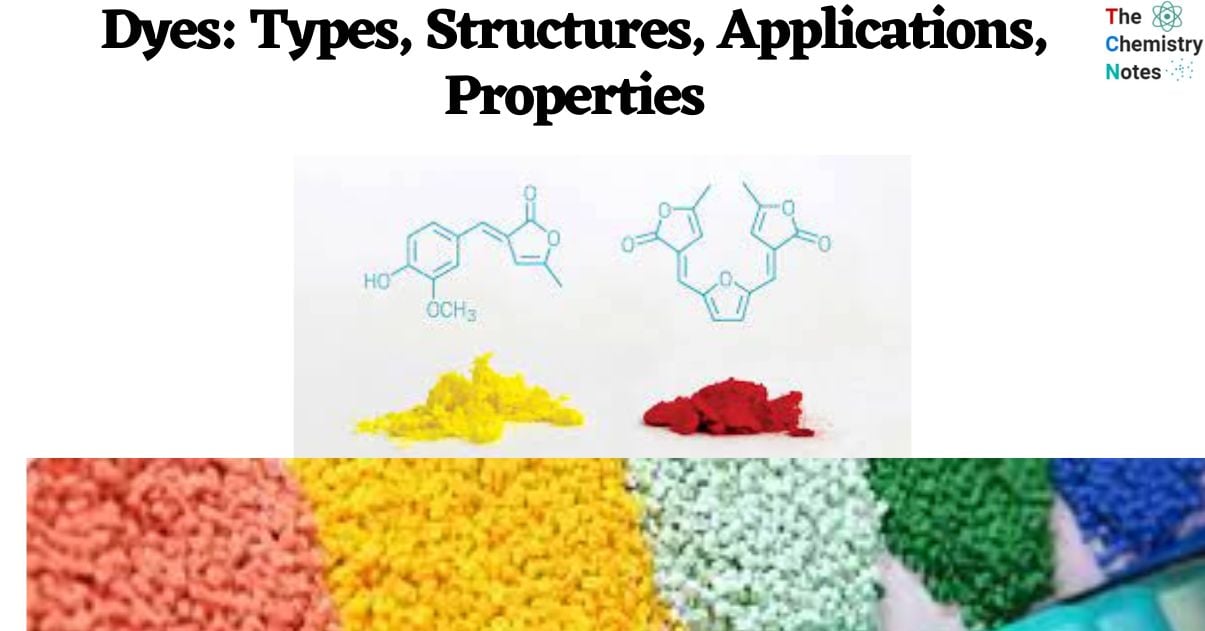
- The majority of acid dyes are sodium salts of organic sulfuric acids. The substance is usually a sulfonic acid salt (D-SO3Na) composed of an aromatic framework with chromogen and solubilizing properties.
- Acidic (anionic) dyes are water-soluble colors that are commonly used for dyeing various fabrics such as wool, silk, nylon, modified rayon, certain modified acrylics, and polyester.
- Cellulosic fibers, which are prone to acid-induced damage, are incompatible with the dyeing process employing this particular set of dyes.
- The chemical composition of the dyes in this class varies, but they all share the common feature of being produced in an acid bath.
- The dyes have brilliant hues and cover a wide range of colors; however, their colorfastness varies.
Properties of Acid Dyes
- Acid dyes are capable of generating a diverse array of vibrant hues.
- They lack a preference for cellulosic fibers. These dyes exhibit a strong affinity for protein and polyamide fibers, making them the primary category of dyes employed in the dyeing processes of wool, silk, and nylon
- From a chemical perspective, acid dyes exhibit similarities to direct dyes in that they yield a colorful anion (DSO3–) and a colorless cation (Na+) upon dissolution. The compounds are commonly denoted as D–SO3Na, with the symbol ‘D’ referring to the chromophoric moiety of the dye molecule.
Applications of Acid Dyes
- Acid dye is a type of dye that is commonly utilized in textile applications under conditions of low pH.
- Primarily, their application is directed toward the coloring of wool rather than cotton textiles.
- Certain acid dyes find application as food colorants.
- Certain substances can also be employed for purposes of marking organelles within the medical domain.
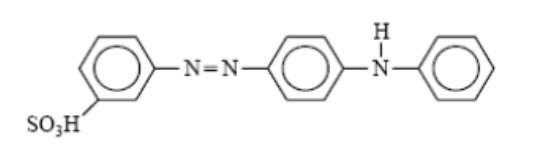
- Basic Dyes
- Basic dyes, which are classified as water-soluble cationic dyes, find their application primarily in acrylic fibers, although certain variants are also utilized in the dyeing processes of wool and silk. Cationic dyes, classified as basic dyes, exhibit remarkable brightness but suffer from inadequate colorfastness, thereby restricting their applicability to cellulosic and protein fibers.
- Basic dyes possess the inherent capacity to proficiently impart color to silk and wool fibers upon immersion in an acidified dye bath. Basic dyes possess the inherent ability to bestow color upon cotton fibers, albeit contingent upon the presence of a mordant, commonly in the guise of a metallic salt.
- The chromophoric segment of the dye molecule possesses a cationic charge. Acrylic fibers exhibit a notable degree of colorfastness when subjected to basic dyes.
- Nylon and polyester fibers, upon undergoing modifications to enhance their affinity for basic dyes, demonstrate remarkable colorfastness properties.
- Mauveine, the inaugural synthetic dye, is classified within this particular category.
Properties of Basic Dyes
- The extraordinary characteristics of fundamental dyes include their exceptional brilliance and color intensity.
- The tinctorial value of basic dyes is noticeably increased.
- The lightfastness and washing speed of the above-mentioned material aren’t as good as they could be. This is because the material doesn’t keep much light and isn’t very resistant to cleaning.
- Basic dyes are used to color knitted fabrics, especially when bright colors are needed that can’t be made with acid dyes. There are a lot of ways that simple dyes can be used to color silk.
Applications of Basic Dyes
- Basic dyes are widely utilized in the dyeing process of various types of fibers, including wool, silk, and acrylic.
- The dye bath is usually mixed with acetic acid to make it easier for the fiber to absorb the color.
- Also, it should be mentioned that these dyes are used to color paper.
- Direct Dye
- Direct dyes, which possess solubility and a strong attraction towards cellulose fibers, are classified as substantial dyes.
- Direct dyes are often synthesized by introducing salt, sodium sulfate, or sodium carbonate into a dye bath that is heated to or close to its boiling point.
- The aqueous solution of dye can exhibit either a neutral pH or a slightly alkaline pH. Cotton, paper, leather, wool, silk, and nylon are all amenable to direct dyeing processes.
- Direct dyes are most effectively utilized in situations where wet cleaning is limited. Direct dyes refer to a category of dyes that undergo development on the fabric after the dyeing process.
- Direct dyes are composed of sodium salts derived from aromatic sulfonic acids, with the predominant chromophore in the majority of these dyes being an azo group.
Properties of Direct Dyes
- Direct dyes have a strong affinity to cellulose and protein fibers, with wool in particular, and are soluble in water.
- Chemically speaking, the dyes are sodium salts of sulfuric acid (DSO3Na).
- The dye molecules undergo dissociation into ions, specifically DSO3– and Na+ when they are dissolved in water. The textile material is subjected to the absorption of colored anions from the dye solution during the dyeing process. Following this, molecule diffusion takes place inside the fiber, which eventually results in its retention or anchoring by physical forces.
Applications of Direct Dyes
- Cellulosic fibers can be dyed directly. A majority of them also dye wool and silk in addition to viscose rayon.
- They don’t dye rayon, acetate, or synthetic fabrics. Because direct dyes may be applied at low temperatures, they are appropriate for dyeing and discharge printing tasks.
- In general, these dyes are used in situations where high wash fastness is not necessary.
Metal Complex Dyes
- Metal complex dyes, also known as pre-metalized acid dyes, belong to a category of dyes that possess unique characteristics. During the process of making the dye, a metal, usually chromium, is added to the dye molecule. This is what causes this effect.
- The integration of metal into the dye molecule increases its affinity for the fiber, which speeds up the dyeing process and results in a comparatively shortened duration. This is because the metal strengthens the dye’s affinity for the fiber.
Properties of Metal Complex Dyes
- The lightfastness rating of metal Complex bi is around 5.
- The washing process is rated on a scale of 4 to 5. The substantial occurrence of van der Waals interactions between the relatively large dye molecules and the macromolecules of the fiber hinders the removal of dye molecules during laundry.
- Apart from covalent bonding, several intermolecular dye-fiber bonds, such as ionic bonds, hydrogen bonds, and coordinate bonds, play a vital role in contributing to this phenomenon.
- The prevalence of chromium in these dyes results in a limited range of relatively dull colors.
Applications of Metal Complex Dyes
- Metal-complex dyes find extensive utility across many applications, including but not limited to wood staining, leather finishing, printing inks for stationery, and coloring agents for metals and plastics. These materials are appropriate for use with wool, silk, and polyamides.
- The metallic elements employed in this study encompass copper, chromium, cobalt, and nickel.
- The fabric treated with metal-complex dyes exhibits favorable light-fastness characteristics. Nonetheless, the speed of the process is also contingent upon the selection of the fiber and the specific dye utilized. Nonetheless, the wet fastness of the fabric is minimal, especially when dealing with darker colors.
- The dyes undergo dyeing processes in conditions of neutral pH, slightly acidic pH, or occasionally severely acidic pH.
Reactive Dye
- Fiber-reactive dyes possess the ability to form chemical bonds with fiber molecules through either addition or substitution reactions. The removal of color is contingent upon its proper application.
- The enduring nature of reactive dyes can be attributed to the covalent bonds they form with natural fibers, rendering them highly stable and resistant to fading or washing out.
- Well-known brands of reactive dyes, such as Procion MX, Cibacron F, and Drimarene K, have the advantage of being easy to use because they can be used at room temperature. Even at elevated temperatures, these substances can be effectively utilized.
- The chromatic properties of colors are characterized by their vividness and commendable resistance to fading. However, it is worth noting that these colors are prone to vulnerability when exposed to chlorine-based bleaching agents, which may result in detrimental effects. Reactive dyes are capable of imparting color to various types of cellulosic fibers such as cotton, flax, and viscose rayon, as well as protein-based fibers like silk, wool, and nylon.
- Reactive dyes are commonly employed in tandem with dispersed dyes for the purpose of dyeing blends of polyester and cellulosic fibers. The industry was first acquainted with them in 1956.
Properties of Reactive Dyes
- Due to the presence of sulfuric acid groups in their molecules, reactive dyes are easily soluble in water.
- In contrast to direct dyes, reactive dye molecules exhibit lower substantivity to cotton and necessitate larger quantities of salt for the process of exhaustion.
- The length of reactive dye molecules is comparatively less than that of direct dyes. Short molecules possess two distinct advantages. The two key factors to consider are the clarity and brightness of the hue, as well as the ease of penetration, which contributes to proper leveling.
- Textile materials that are dyed with reactive dyes exhibit satisfactory to excellent levels of light and washing fastness.
Applications of Reactive Dyes
- While several reactive dyestuffs have been specifically modified for wool dyeing, their main use is for the coloring of cotton, linen, and viscose rayon fibers.
- Cold-water fiber reactive dyes work well with a variety of textile components, including cotton, silk, jute, rayon, and hessian, making them suitable for dyeing.
- Reactive dyes are employed in situations where there is a need for vibrant coloring with exceptional levels of colorfastness and resistance to fading during washing.
- The technique of cold dying is widely employed in the practice of batik.
- While several reactive dyestuffs have been specifically engineered for wool dyeing, their primary application is in the dyeing of cotton, linen, and viscose rayon fibers.
Water Insoluble Dyes
(i) Disperse Dyes
- The initial inception of disperse dyes was primarily geared towards the chromatic alteration of acetate fibers. Hydrophobic fibers exhibit a minimal propensity for forming chemical bonds with water-soluble dyes. Disperse dyes are dyes that are very hard to dissolve in water, so they are finely spread out.
- The dimensions of dispersed dye particles fall within the approximate range of 0.5 to 1 micrometer. A new way to give color to fibers that don’t like water has been found. It involves dispersing pigmented organic compounds in aqueous media with the help of a surfactant.
- The fibrous materials that exhibit the highest affinity for dispersed dyes encompass cellulose acetate, polyester, acrylic, and nylon. These fibers demonstrate commendable exceptional colorfastness properties. From a chemical standpoint, it is worth noting that disperse dyes encompass a diverse array of classes, including but not limited to azo, anthraquinone, methylene, and diphenylamine.
- The dyes typically possess functional groups such as hydroxyl (-OH), nitro (-NO), cyano (-CN), halogen, and amine groups while conspicuously lacking any polar moieties.
(ii) Vat Dye
- Vat dyes possess inherent insolubility in aqueous solutions, hence preventing them from immediately providing coloration to fibers. However, the reduction in the alkaline solution leads to the creation of a water-soluble complex composed of alkali metal salt and color. The chemical, when in its leucoform, demonstrates a pronounced affinity for the textile fiber.
- The inaugural instance of a synthetic indigo dye, which made its debut in the industrial sphere in the year 1896, pertains to this particular category.
- The utilization of dyes can be traced back to ancient times, as they represent one of the earliest instances of natural coloring agents derived from various sources such as botanical specimens (including plants and stems), entomological specimens (insects), and zoological specimens (animals).
- Vat dyes afford textile materials with unparalleled colorfastness, surpassing all other dyes commonly employed in this domain.
- The etymology of the term “vat dyes” can be attributed to the historical practice of utilizing fermentation techniques within wooden containers, colloquially referred to as “vats,” to apply indigo, the primary constituent of this dye category, onto textile materials.
- These substances find their primary application in the process of imparting color to cotton work garments, athletic attire, printed textiles, drapery materials, and fabrics composed of a blend of cotton and polyester.
(iii) Sulfur Dye
- A lot of black, blue, maroon, olive, and green colors with medium to deep depths are made with sulfur dyes.
- Sulfur dyes, owing to their economical nature, are widely utilized in the dyeing process of cellulosic fibers and their amalgamations, specifically polyester and cotton yarn, within a package or hank dyeing apparatus.
- The fastness of sulfur dyes varies greatly from one color to the next. For example, the light fastness of yellow dyes is almost 3, while that of black dyes is about 7. In general, the speed of washing is good.
- The inherent capacity for bleaching is notably deficient, save for a select few dyes. The aforementioned dyes can be procured in various states of matter, including powder, pre-reduced powder, grains, paste, and liquid.
(iv) Azoic Dye
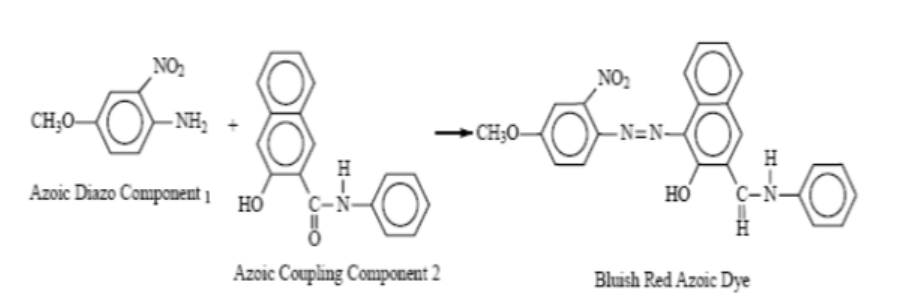
- Azoic dyes, in essence, are not preexisting dyes, but rather they are synthesized within the substrate through the utilization of two distinct constituents, namely naphthol, and bases. These constituents are commonly referred to as “coupling components” and “diazo components,” respectively.
- Due to the necessity of attaining exceedingly elevated temperatures, which necessitates the utilization of frozen water in the process of applying azoic dyes, this particular category of dyes has acquired the moniker of “ice colors”.
(v) Ingrain Dye
- The chromatic pigment resulting from the chemical amalgamation of the two achromatic constituents manifests insolubility in aqueous solutions, thereby showcasing exceptional resistance to moisture-based procedures.
- Water-soluble components interact chemically with one another in an aqueous environment to produce the insoluble dye. This phenomenon has a big impact on how the reaction manifests itself during the solution phase.
- When the pigment forms in the bath, it leaves a layer on the material’s surface that doesn’t stick very well. This makes the material less resistant to pressing.
- But the process of dying tries to get the insoluble azoic color into the structure of the fiber. Because of this, azoic dyes are sometimes called “ingrain dyes.”
The dyeing process plays a pivotal role in the prosperous exchange of textile commodities. The techniques employed in the process of dying have exhibited minimal evolution throughout the course of history. In essence, water serves as a medium for the cleansing, dyeing, and application of chemical agents onto textiles, while also facilitating the subsequent rinsing of the treated fibers or fabrics.
References
- https://www.britannica.com/technology/dye
- https://chemistnotes.com/organic/dye-definition-classification-example/
- https://www.askmattrab.com/notes/1343-Dyes-and-Drugs
- https://textilelearner.net/different-types-of-dyes-with-chemical-structure/
- https://www.meghmaniglobal.com/different-types-of-dyes-with-chemical-structure/
- Kumar Samanta, A., S. Awwad, N., & Majdooa Algarni, H. (Eds.). (2020). Chemistry and Technology of Natural and Synthetic Dyes and Pigments. IntechOpen. doi: 10.5772/intechopen.83199

Dear Madam,
May I know that MB(methylene blue) dye degraded higher than XyO (Xylenol orange)dye. What may be the reason for the restrictive behavior of XyO dye from degradation?