An E1 mechanism reaction, also known as unimolecular elimination, mirrors the SN1 reaction and stands in contrast to electrophilic addition. Typically a two-step process, it commonly involves tertiary alkyl halides, though select secondary ones may also participate. E1 elimination occurs primarily in allylic or benzylic positions when the leaving group is attached to a primary carbon. Weaker bases such as water and alcohol typically promote E1 reactions, exemplified by the formation of 2-Methyl propene from 2-Bromo-2-methyl propene in the presence of aqueous ethanol at 25°C.
Interesting Science Videos
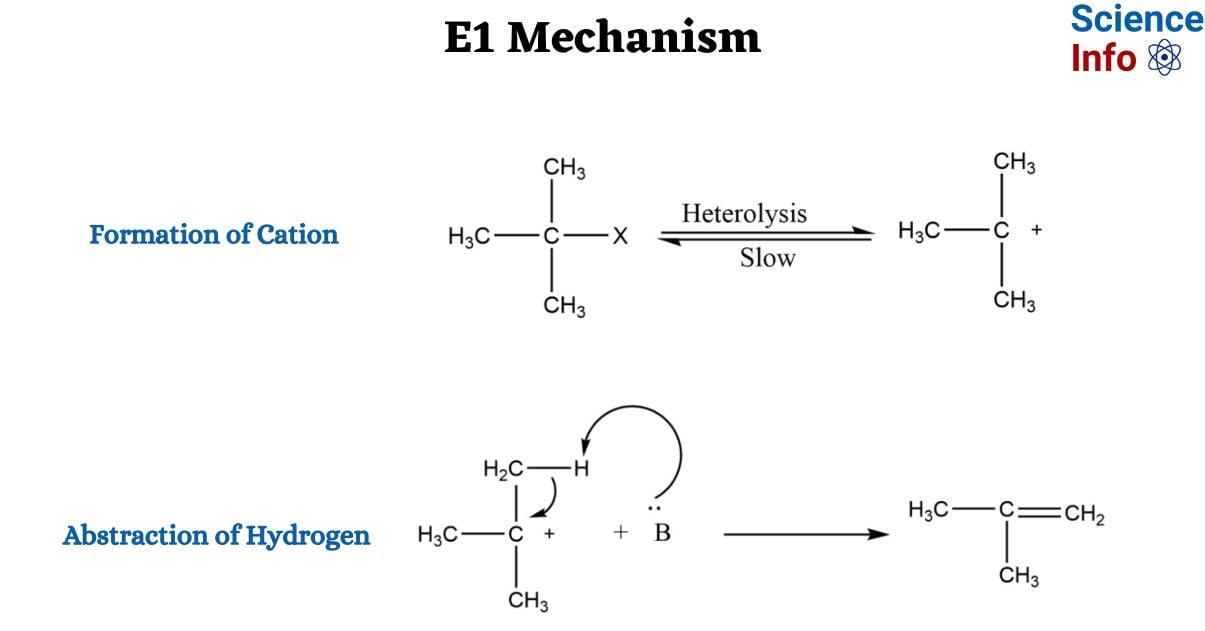
E1 Mechanism
Formation of Cation
Initiated by the departure of a leaving group, a carbocation intermediate is formed.

Abstraction of Hydrogen
A base removes a nearby proton, filling the carbocation’s vacant p orbital with two electrons, resulting in the creation of a new p bond. This step, also involving the leaving group or another basic species, characterizes E1 reactions as β-elimination reactions.

E1 reactions often yield a mixture of alkene products, following Zaitsev’s rule, favoring the most substituted alkene product. Both cis and trans alkenes can be produced, with trans alkenes generally being more stable.
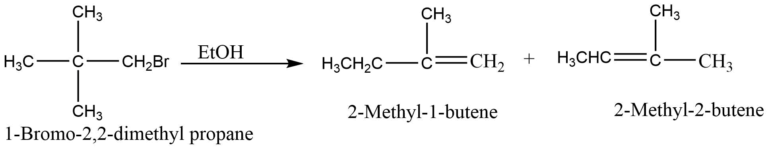
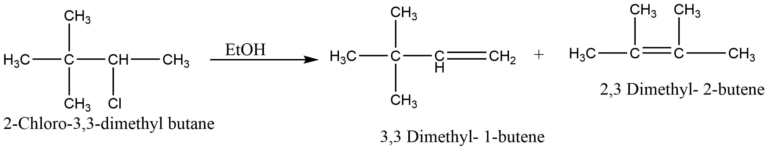
Since a carbocation intermediate is involved, E1 reactions may undergo carbocation rearrangement for increased stability. For example, dehydrohalogenation of 2-chloro-3,3-dimethyl butane predominantly yields 2,3 dimethyl-2-butene.
Kinetics of E1 Mechanism
- The reaction adheres to first-order kinetics.
- First-order with respect to the substrate and zero-order with respect to the base, making the rate dependent solely on substrate concentration.
Stereochemistry of E1 Reaction
E1 reactions lack geometric requirements due to the separate loss of hydrogen and halide in different steps, favoring the more stable product according to Zaitsev’s rule.
Reactivity and Orientation
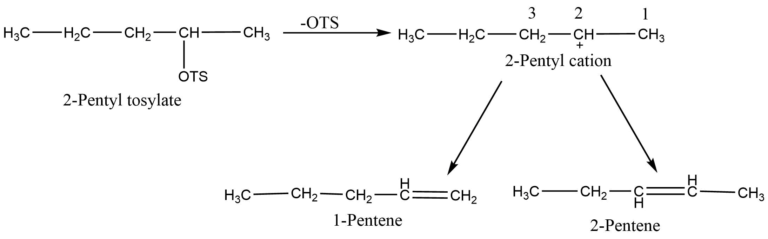
The E1 reactions are regioselective. Saytzeff orientation is heavily followed by elimination processes utilizing the E1 mechanism. When many alkenes can be synthesized, the product with the most branching is preferred. A disubstituted alkene is preferred over a monosubstituted one. For example, the primary product of 2-pentyl tosylate is 2-pentene.

Relative reaction rates of several processes continue to define the orientation and reactivity. The rate of step first determines how quickly the substrate reacts. However, which β -proton leaves the carbocation from second step faster determines which alkene is produced. For example, the 2- pentyl cation can lose either a proton from 2- pentene or a proton from C1 to form 1 pentene. There is competition and more 2- pentene is obtained because 2- pentene is formed faster.
The alkyl halide’s substitution pattern has a significant impact on the rate of reaction. As a result, the rate increases in the following order: tertiary (fastest) > secondary >> primary (slowest). The carbonium ion generated in the first step has the greatest stability, which allows the reaction to be completed, giving rise to the tertiary alkyl halide’s greatest reactivity. The most stable carbonium ion is a tertiary one, followed by secondary and primary. I > Br > Cl > F is the order of the reactivity with various halogens for a particular alkyl group.
Factors Affecting Reaction Rate
- Nature of the Substrate: Highly substituted alkyl halides, forming more stable carbocation intermediates, favor E1 reactions.
- Basicity and Concentration of Base: Weaker bases like water and alcohol generally promote E1 reactions.
- Nature of the Leaving Group: Less basic and more polarizable leaving groups enhance substrate reactivity.
- Nature of the Solvent: Increasing solvent polarity, especially in hydroxylic solvents with low nucleophilicity, favors E1 reactions.
- Effect of Temperature: E1 reaction rates increase with temperature.
Characteristics of E1 mechanism
- Two-step reaction process exhibiting first-order kinetics.
- Reaction rate depends solely on substrate concentration.
- Highly substituted alkyl halides react faster.
- Favored by weak bases like water and alcohol.
- Better leaving groups increase reaction rate.
- The presence of a leaving group capable of stabilizing the ionic intermediate enhances the E1 reaction.
Video on E1 mechanism
References
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- https://www.masterorganicchemistry.com/2012/09/19/the-e1-reaction/
- http://www.lscollege.ac.in/sites/default/files/e-content/Elimination_reaction.pdf
- https://kpu.pressbooks.pub/organicchemistry/chapter/8-2-e1-reactions/.
- https://www.studysmarter.co.uk/explanations/chemistry/organic-chemistry/e1-elimination/
- https://chemicalnote.com/elimination-reaction-e1-and-e2-reaction-examples-mechanism-orientation-and-reactivity/#google_vignette
- https://www.chem.ucalgary.ca/courses/353/Carey5th/Ch05/ch5-5.html.
- https://www.masterorganicchemistry.com/2012/09/19/the-e1-reaction/.
- https://home.iitk.ac.in/~madhavr/CHM102/Lec13.pdf
- https://www.chemistrysteps.com/the-e1-mechanism/

