Electron orbitals are areas within the atom where electrons are most likely to be located. Electrons are not merely floating around within the atom; rather, they are confined within electron orbitals.
Interesting Science Videos
What is an Electron Orbital?
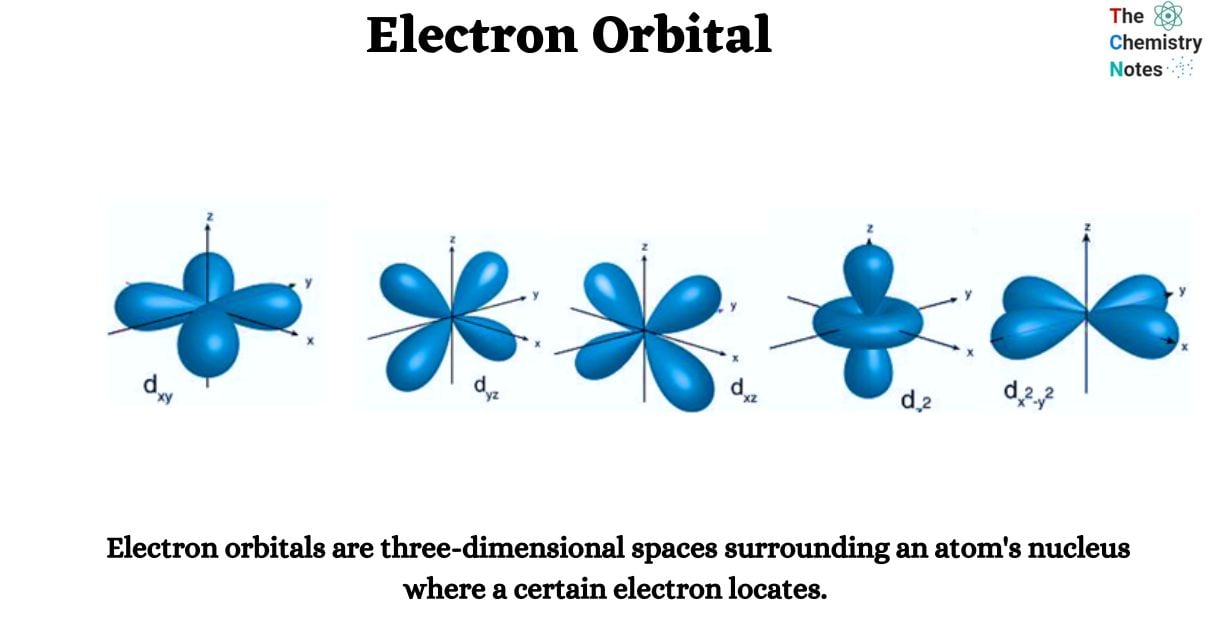
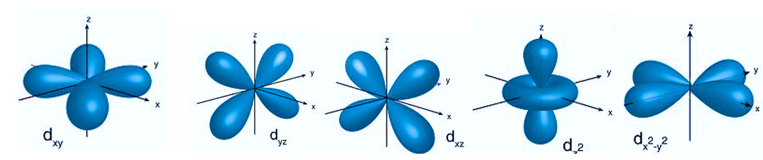
Electron orbitals are three-dimensional spaces surrounding an atom’s nucleus where a certain electron locates. Each electron orbital may accommodate two electrons. They are sometimes referred to as atomic orbitals. The form of an atomic orbital depends on the amount of electrons in the atom.
An orbital, in its most exact description, is a mathematical function that specifies the placement of an electron in an atom based on probability. The term “atomic orbital” often refers to the region of space in which an electron might conceivably exist depending on the mathematical function for that orbital.
Quantum Numbers and Atomic Orbitals
- The quantum numbers n, l, and m are used to categorize atomic orbitals and represent the electron’s energy, angular momentum, and an angular momentum vector component.
- Each orbital is defined by a distinct set of quantum numbers (n, l, and m) and may accommodate up to two electrons, each with a distinct spin quantum number.
- The s orbital, p orbital, d orbital, and f orbital are simple names for orbitals with angular momentum quantum numbers l = 0, 1, 2, and 3. As a result, these names describe electron configurations and indicate orbital shape.
- The total number of electrons occupying a full set of the s, p, d, and f atomic orbitals, respectively, naturally gives rise to the recurring periodicity of the blocks of 2, 6, 10, and 14 elements inside periodic table sections.
- Degenerate orbitals are those that have the same energy level or same energy level. The 2p orbital is an example: 2px has the same energy level as 2py. This idea becomes much more significant when dealing with molecular orbitals. According to the Pauli exclusion principle, no two electrons may have the same precise orbital arrangement; in other words, the identical quantum numbers. However, the electron can exist in either spin up (ms = +1/2) or spin down (ms = -1/2) states.
Orbits and Orbitals
An orbital is a probable location within an atom with the highest density of electron presence. An orbit, on the other hand, just exists in a body of a particular mass. An orbital is found in an electron and an atom.
Orbit: An orbit is a well-defined circular route that electrons revolve along around the nucleus. It’s also known as a shell. It is represented by the letter “n,” which represents the main quantum number. Electrons must either absorb or release energy to travel from one orbit to another. Assume that when an electron goes from a higher energy level to a lower energy level, it must release a specific amount of energy and absorb energy when it moves from a lower energy level to a higher energy level.
Orbital: An orbital is a zone of uncertainty in which an electron’s presence or placement is most likely. As a result, the region surrounding the nucleus is a three-dimensional depiction of the orbital. The orbital’s energy also allows it to take on a variety of forms.
Video on Electron Orbitals
References
- R. S. Mulliken, Electronic Structures of Molecules and Valence. II General Considerations, Physical Review, vol. 41, pp. 49-71 (1932)
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals
- W. Heitler, Elementary Wave Mechanics, 2nd edition (1956) Clarendon Press, Oxford, UK.
- Griffiths, David (1995). Introduction to Quantum Mechanics. Prentice Hall. pp. 190–191. ISBN 978-0-13-124405-4.
- Feynman, Richard; Leighton, Robert B.; Sands, Matthew (2006). The Feynman Lectures on Physics – The Definitive Edition, Vol 1 lect 6. Pearson PLC, Addison Wesley.
- https://chemistrytalk.org/electron-orbitals-and-shapes/

