Electronegativity is the tendency of an atom in a molecule to attract the shared pair of electrons to itself.
It essentially represents the total outcome of the tendency of atoms in various elements to attract bond-forming electron pairs. We measure electronegativity at various scales. Linus Pauling devised the most widely used scale to draw the shared pair of electrons towards itself, which is known as electronegativity.
Electronegativity ranges from zero to four.
Interesting Science Videos
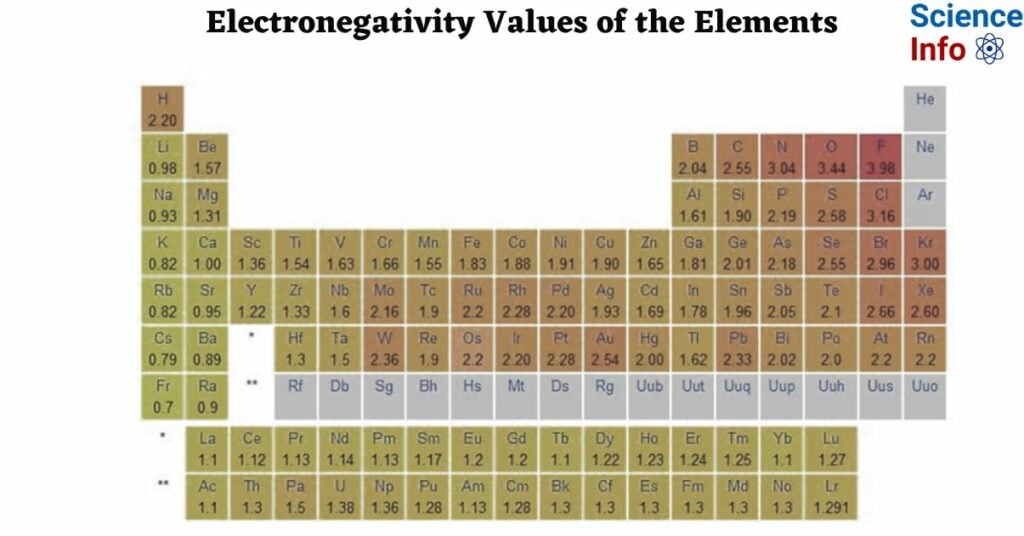
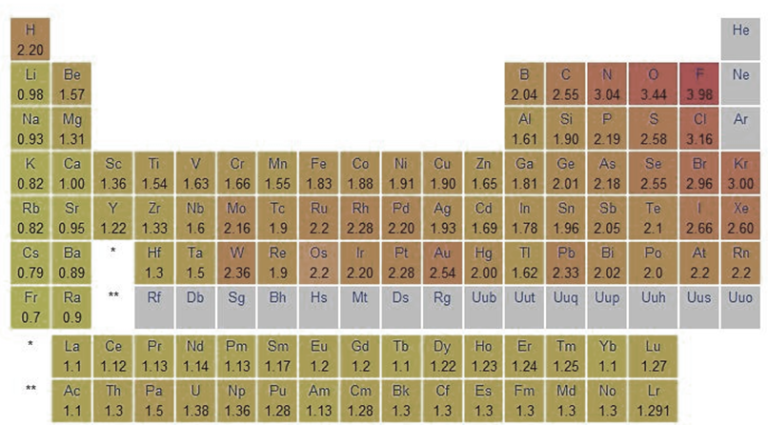
Electronegativity Values of the Elements
| Atomic Number | Element | Symbol | Electronegativity |
| 1 | Hydrogen | H | 2.20 |
| 2 | Helium | He | 0 |
| 3 | Lithium | Li | 0.98 |
| 4 | Beryllium | Be | 1.57 |
| 5 | Boron | B | 2.04 |
| 6 | Carbon | C | 2.55 |
| 7 | Nitrogen | N | 3.04 |
| 8 | Oxygen | O | 3.44 |
| 9 | Fluorine | F | 3.98 |
| 10 | Neon | Ne | 0 |
| 11 | Sodium | Na | 0.93 |
| 12 | Magnesium | Mg | 1.31 |
| 13 | Aluminum | Al | 1.61 |
| 14 | Silicon | Si | 1.90 |
| 15 | Phosphorus | P | 2.19 |
| 16 | Sulfur | S | 2.58 |
| 17 | Chlorine | Cl | 3.16 |
| 18 | Argon | Ar | 0 |
| 19 | Potassium | K | 0.82 |
| 20 | Calcium | Ca | 1.00 |
| 21 | Scandium | Sc | 1.36 |
| 22 | Titanium | Ti | 1.54 |
| 23 | Vanadium | V | 1.63 |
| 24 | Chromium | Cr | 1.66 |
| 25 | Manganese | Mn | 1.55 |
| 26 | Iron | Fe | 1.83 |
| 27 | Cobalt | Co | 1.88 |
| 28 | Nickel | Ni | 1.91 |
| 29 | Copper | Cu | 1.90 |
| 30 | Zinc | Zn | 1.65 |
| 31 | Gallium | Ga | 1.81 |
| 32 | Germanium | Ge | 2.01 |
| 33 | Arsenic | As | 2.18 |
| 34 | Selenium | Se | 2.55 |
| 35 | Bromine | Br | 2.96 |
| 36 | Krypton | Kr | 3.00 |
| 37 | Rubidium | Rb | 0.82 |
| 38 | Strontium | Sr | 0.95 |
| 39 | Yttrium | Y | 1.22 |
| 40 | Zirconium | Zr | 1.33 |
| 41 | Niobium | Nb | 1.6 |
| 42 | Molybdenum | Mo | 2.16 |
| 43 | Technetium | Tc | 1.9 |
| 44 | Ruthenium | Ru | 2.2 |
| 45 | Rhodium | Rh | 2.28 |
| 46 | Palladium | Pd | 2.20 |
| 47 | Silver | Ag | 1.93 |
| 48 | Cadmium | Cd | 1.69 |
| 49 | Indium | In | 1.78 |
| 50 | Tin | Sn | 1.96 |
| 51 | Antimony | Sb | 2.05 |
| 52 | Tellurium | Te | 2.1 |
| 53 | Iodine | I | 2.66 |
| 54 | Xenon | Xe | 2.6 |
| 55 | Cesium | Cs | 0.79 |
| 56 | Barium | Ba | 0.89 |
| 57 | Lanthanum | La | 1.10 |
| 58 | Cerium | Ce | 1.12 |
| 59 | Praseodymium | Pr | 1.13 |
| 60 | Neodymium | Nd | 1.14 |
| 61 | Promethium | Pm | 1.13 |
| 62 | Samarium | Sm | 1.17 |
| 63 | Europium | Eu | 1.2 |
| 64 | Gadolinium | Gd | 1.2 |
| 65 | Terbium | Tb | 1.22 |
| 66 | Dysprosium | Dy | 1.23 |
| 67 | Holmium | Ho | 1.24 |
| 68 | Erbium | Er | 1.24 |
| 69 | Thulium | Tm | 1.25 |
| 70 | Ytterbium | Yb | 1.1 |
| 71 | Lutetium | Lu | 1.27 |
| 72 | Hafnium | Hf | 1.3 |
| 73 | Tantalum | Ta | 1.5 |
| 74 | Tungsten | W | 2.36 |
| 75 | Rhenium | Re | 1.9 |
| 76 | Osmium | Os | 2.2 |
| 77 | Iridium | Ir | 2.2 |
| 78 | Platinum | Pt | 2.28 |
| 79 | Gold | Au | 2.54 |
| 80 | Mercury | Hg | 2.00 |
| 81 | Thallium | Tl | 1.62 |
| 82 | Lead | Pb | 2.33 |
| 83 | Bismuth | Bi | 2.02 |
| 84 | Polonium | Po | 2.0 |
| 85 | Astatine | At | 2.2 |
| 86 | Radon | Rn | 0 |
| 87 | Francium | Fr | 0.7 |
| 88 | Radium | Ra | 0.89 |
| 89 | Actinium | Ac | 1.1 |
| 90 | Thorium | Th | 1.3 |
| 91 | Protactinium | Pa | 1.5 |
| 92 | Uranium | U | 1.38 |
| 93 | Neptunium | Np | 1.36 |
| 94 | Plutonium | Pu | 1.28 |
| 95 | Americium | Am | 1.3 |
| 96 | Curium | Cm | 1.3 |
| 97 | Berkelium | Bk | 1.3 |
| 98 | Californium | Cf | 1.3 |
| 99 | Einsteinium | Es | 1.3 |
| 100 | Fermium | Fm | 1.3 |
| 101 | Mendelevium | Md | 1.3 |
| 102 | Nobelium | No | 1.3 |
Electronegativity values missing in the table are not known because reliable experimental values for electronegativity are not available for those elements. It is often determined using a variety of experimental approaches, such as bond energies and dipole moments. However, for some elements, particularly those that are scarce, highly unstable, or difficult to work with, acquiring correct experimental data might be problematic. As a result, electronegativity values for some elements may not be accessible or must be calculated using theoretical calculations or extrapolations from comparable elements.
Trends of Electronegativity in Periodic Table
- In general, E.N. rises as you cross a period in the periodic table from left to right. As you move down a group, it gets smaller.
- Accordingly, the top right of the periodic table shows the elements with the highest E.N. potential, while the bottom left shows the elements with the lowest electronegative potential.
- The periodic table shows a correlation between electronegativity and ionization energy. Low electronegativities are typical of elements with low ionization energies. These atoms’ nuclei don’t pull on electrons very strongly.
- The E.N. values of elements tend to be high when their ionization energies are high. Electrons experience a strong pull from the atomic nucleus.
- IA alkali metal has the lowest E.N. value and VIIA halogen has the highest value over a given time period.
- On the periodic table, F has the highest E.N. and Cs has the lowest.
- Cs(55) should have a higher E.N. than Fr(87), but it does not. This is because the nuclear charge of Fr has increased by +32 units, making the effective nuclear charge comparatively high.
References
- https://scienceinfo.com/electronegativity/
- https://byjus.com/chemistry/electronegativity/
- https://sciencenotes.org/list-of-electronegativity-values-of-the-elements/
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Chem
- Mullay, J. (1987). “Estimation of atomic and group electronegativities”. Electronegativity. Structure and Bonding. Vol. 66. pp. 1–25. ISBN 978-3-540-17740-1. doi:10.1007/BFb0029834
