Electronegativity is a key component of chemists’ intuitive approach to understanding nature, which distinguishes them from other physical scientists. The concept of electronegativity is practically a direct consequence of fundamental ideas of chemical bonds and sharing of electrons in modern chemistry, Consequently, the ability of an atom in a molecule to attract electrons is described as Pauling’s contribution. It is understandable why it has been so helpful given how closely it relates to fundamental ideas. Years of research have led to at least one apparent conclusion: there is something about atoms that causes unequal electron sharing. As a result, the concept of electronegativity has changed from the original concept of assigning a single value to each atom to the current formulation, which involves a range of values that depend on the state of the atom in the molecule.
Interesting Science Videos
Electronegativity- Definition
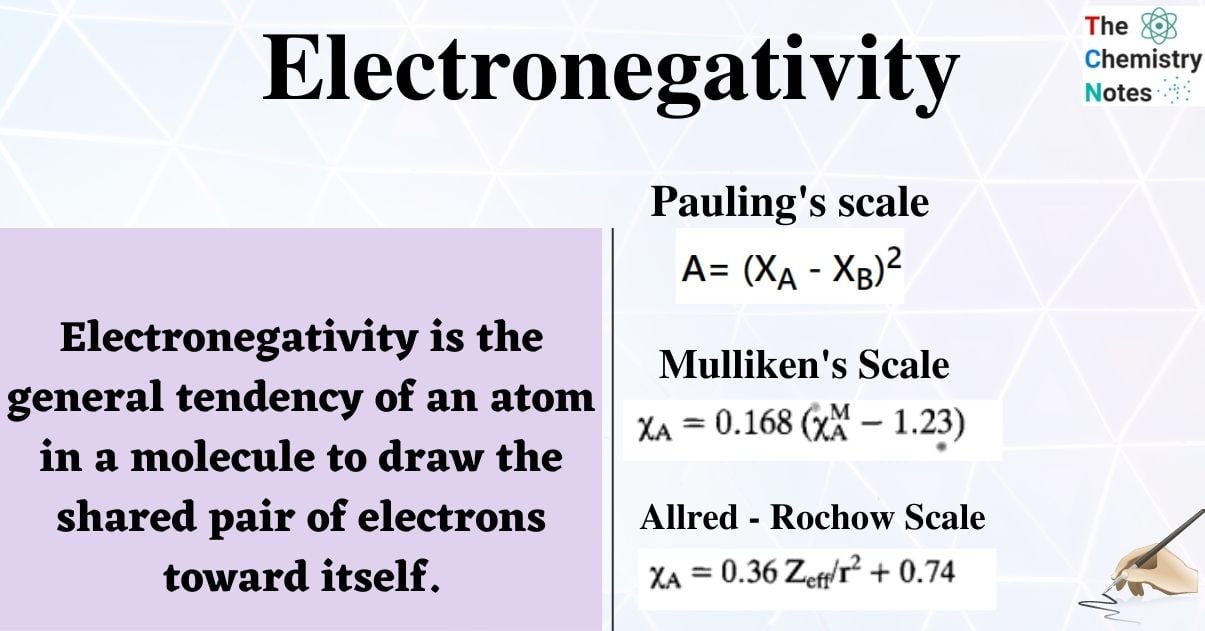
Electronegativity(E.N) is the general tendency of an atom in a molecule to draw the shared pair of electrons toward itself.
It is defined as an atom’s ability to attract electrons to itself in a chemical bond. It is the chemical variable that determines the kind of bonds that are formed between atoms.
An atom’s ability to attract the electrons of a bond increases with electronegativity, a property of an atom. The electrons in a covalent bond are shared equally by two bonded atoms if their E.N values are the same. Typically, the more electronegative atom attracts the electrons in a chemical bond more so than the other atom. Consequently, a polar covalent bond is created. The electrons aren’t shared at all if the E.N values are very dissimilar. Ionic bonds are created when one atom essentially steals the other atom’s bond electrons.
Many definitions of E.N exist, and they can be roughly categorized as either spectroscopic (defined for isolated atoms) or thermo-chemical (characterizing bond energies and heats of formation of compounds).
History
- Contrary to popular belief, the concept of electronegativity existed before Linus Pauling’s invention in 1932.
- Hans Peter Jörgen Julius Thomsen, a thermochemist from Denmark, proposed an electropositive and electronegative periodic table in 1895.
- Before Jöns Jacob Berzelius gave it a formal name in 1811, Avogadro and other chemists studied E.N.
- Hans Peter Jörgen Julius Thomsen, a thermochemist from Denmark, proposed an electropositive and electronegative periodic table in 1895:
- But Linus Pauling’s The Nature of the Chemical Bond, published in 1932, gave the idea of E.N. a quantitative foundation.
Electronegativity Scales
The following are a few arbitrary scales for the quantitative measurement of electronegativities.
Pauling’s scale
Pauling electronegativity is based on the empirical observation that bonds between atoms with a large E.N. difference tend to be stronger than those where the difference is small. This is done by using bond energies. Although it lacks a solid theoretical foundation, this scale was historically the first to be created. Pauling used the E.N. of fluorine (4) as an assumption and extrapolated this value to determine the E.N. of other elements.
If A is the difference between these two energies than Pauling’s famous E.N. postulate is given by
A= (ΧA – ΧB)2
where the E.N. of atoms A and B is represented by XA, XB and vice versa. Since Pauling discovered that these and not the A were additive, it should be noted that the E.N. difference is related to (rather than A). Due to the accessibility of thermochemical data, he was able to value a significant number of atoms.
Mulliken’s electronegativity
According to Mulliken, an element’s electronegativity is determined by averaging out its ionization potential and electron affinity. Mulliken electronegativity, which highlights the significance of two potential outcomes in bond formation, losing an electron or gaining one, is the average of an atom’s first ionization energy and its electron affinity. The scale has the advantage that E.N. values can be calculated for other electron configurations, including polyatomic fragments, in addition to the ground states of atoms.
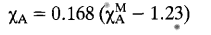
Although Pauling’s scale of reference is still the preferred one, Mulliken’s method has much more theoretical backing. This is primarily due to the lack of data necessary to calculate Mulliken electronegativity values for many atoms prior to the early 1960s. For the vast majority of interesting cases involving the transition elements, there is still a dearth of data. Therefore, even today, this electronegativity measurement is not very useful for this significant group of atoms.
The notion that this scale provides the best representation of the electronegativity concept, however, appears to have a lot of support. This is more so in terms of its theoretical content than its quantitative usefulness. It is possible to consider Mulliken’s theories as being either derived from or highly dependent on the vast majority of current theoretical work in E.N. .
Allred – Rochow electronegativity
According to Allred-Rochow, the force that an atom’s nucleus applies to its valence electrons is known as electronegativity. The Mulliken scale is only applicable to monovalent atoms. The relationship between Allred-Rochow electronegativity and Zeff/r2, where Zeff is the effective nuclear charge of the valence orbitals, and r is the atom’s covalent radius is the value is proportional to the nucleus’s actual electrostatic attraction to valence electrons, which is masked by electrons in the inner shell.

It starts by introducing the concept of force into theory of electronegativity. This seems to be straightforward to comprehend and modify, and it appears to be quite consistent with Pauling’s verbal definition. Second, it demonstrates the significance of the notion that usefulness is achieved by maintaining calculational simplicity.
Additionally, it unlocked the greater potential for studying more elements than previously. The Allred-Rochow scale is likely the one that is utilized the most frequently, excluding Pauling’s. electronegativity measurement.
It is predicated on the straightforward premise that the force of attraction between the screened nucleus and an electron at the covalent radius determines an atom’s electronegativity.
Factors Affecting Electronegativity
- Atomic Size: As atomic size increases, electronegativity decreases. Atoms that are smaller have higher electronegativity than larger ones.
- Nuclear Charge: As the nuclear charge rises, so does the electronegativity.
- Screening Effect: As the screening effect rises, the electronegativity falls.
- Oxidation State: As the positive oxidation state rises, the electronegativity rises as well.
- Hybridization: For the same element, hybridization changes the electronegativity. It decreases as the s character of the hybrid orbitals increases. For instance, sp has more s-character, or more EN.
- Electronic Configuration: Atoms with nearly filled electron shells typically exhibit higher electronegativity than do those with sparsely occupied electron shells. Inert gas elements (have no electronegativity because their outer shells are entirely filled).
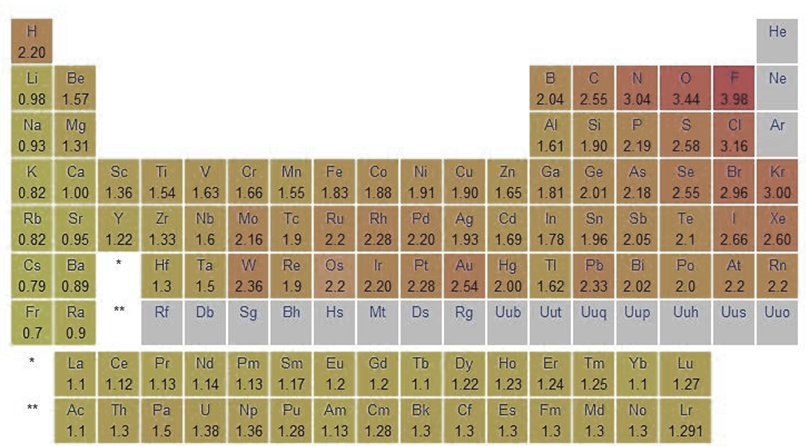
Trends of Electronegativity in Periodic Table
- In general, E.N. rises as you cross a period in the periodic table from left to right. As you move down a group, it gets smaller.
- Accordingly, the top right of the periodic table shows the elements with the highest E.N. potential, while the bottom left shows the elements with the lowest electronegative potential.
- The periodic table shows a correlation between electronegativity and ionization energy. Low electronegativities are typical of elements with low ionization energies. These atoms’ nuclei don’t pull on electrons very strongly.
- The E.N. values of elements tend to be high when their ionization energies are high. Electrons experience a strong pull from the atomic nucleus.
- IA alkali metal has the lowest E.N. value and VIIA halogen has the highest value over a given time period.
- On the periodic table, F has the highest E.N. and Cs has the lowest.
- Cs(55) should have a higher E.N. than Fr(87), but it does not. This is because the nuclear charge of Fr has increased by +32 units, making the effective nuclear charge comparatively high.
References
- Jolly, William L. (1991). Modern Inorganic Chemistry (2nd Edn.). New York: McGraw-Hill. ISBN 0-07-112651-1. pp. 71–76.
- https://byjus.com/chemistry/electronegativity/
- https://chemistrytalk.org/electronegativity-chart-trends/
- https://alevelchemistry.co.uk/definition/electronegativity/’
