The simplest formula of a compound, which represents the whole number ratio of atoms of different elements present in a compound or a class of compounds, is called the empirical formula.
The proportion of distinct elements in a series of compounds is also represented by an empirical formula. It may not accurately reflect the number of atoms in a molecule. CH2O, for example, is the empirical formula of simple carbohydrate monosaccharides, whereas CH2 is the empirical formula of alkenes with a double bond in the molecule.
The C:H:O ratio in monosaccharides is 1:2:1, whereas the C:H ratio in typical alkenes is 1:2. The chemical formula that reveals the precise number of atoms contained in a molecule is the substance’s molecular formula. For example, benzene has the chemical formula C6H6, but sucrose has the formula C12H22O11.
Interesting Science Videos
Relation Between Empirical and Molecular Formula
An empirical formula is related to the molecular formula as follows.
Molecular formula = n x empirical formulawhere, ‘n’ is an integer i.e. n = 1,2,3 and so on. The value of n is calculated by knowing the molecular weight and empirical formula weight, i.e.
n = (mol.weight/empirical formula weight)where empirical formula weight is the sum of all the atoms present in the empirical formula.
For example,
| Empirical Formula | Value of n | Molecular formula |
|---|---|---|
| 1 | 1 x CH2O = HCHO | |
| 2 | 2 x CH2O = C2H4O2 | |
| 3 | 3 x CH2O = C3H6O3 | |
| 4 | 4 x CH2O = C4H8O3 | |
| CH2O | 5 | 5 x CH2O = C5H10O5 |
| 6 | 6 x CH2O = C6H12O6 | |
| 7 | 7 x CH2O = C7H14O7 | |
| 8 | 8 x CH2O = C8H16O8 |
Determining the Empirical Formula
An empirical formula is derived from the percentage composition and atomic weight of the elements. The following steps are used in the derivation of the empirical formula:
- Determine the percentage composition of a compound experimentally or calculate the percentage of each element as usual from the given data.
- Make sure that the total percentage of all the elements is 100. If the total of the percentages of the components is less than 100, the proportion of oxygen is insufficient. If oxygen is already present, add the deficiency quantity is the percentage of oxygen; otherwise, assume that oxygen is present in the compound with a deficient proportion.
- Divide the proportion of all elements by their atomic weight to get the number of moles.
- To calculate the number of moles, divide the total number of moles by the smallest mole. If the ratio of the number of moles obtained is not a whole number, convert it to the nearest whole number by multiplying by an appropriate amount, or round off figures for extremely slight differences.
- The whole number of moles of the element indicates the ratio of atoms of distinct elements present, which is represented by the empirical formula. The empirical formula refers to the ratio of atoms of different elements. For example, if the carbon, hydrogen, and oxygen atom ratios are 1:2:1, the empirical formula is CH2O.
Molecular Formula
- The molecular formula, which is derived from molecules, represents the total number of individual atoms that comprise the molecule of a chemical.
- A subscript in a molecular formula reports the precise number of each type of atom in a compound’s molecule.
- Molecular formulas are allocated gram molecular masses that are simple whole-number multiples of the corresponding empirical formula mass.
Difference Between Molecular and Empirical Formula
| Empirical Formula | Molecular Formula |
|---|---|
| An empirical formula is the simplest whole-number ratio of the different atoms in a molecule. | The molecular formula specifies the number of distinct types of atoms contained in a chemical molecule. |
| Example: Empirical formula of acetylene is CH. | Example: Molecular formula of acetylene is C2H2. |
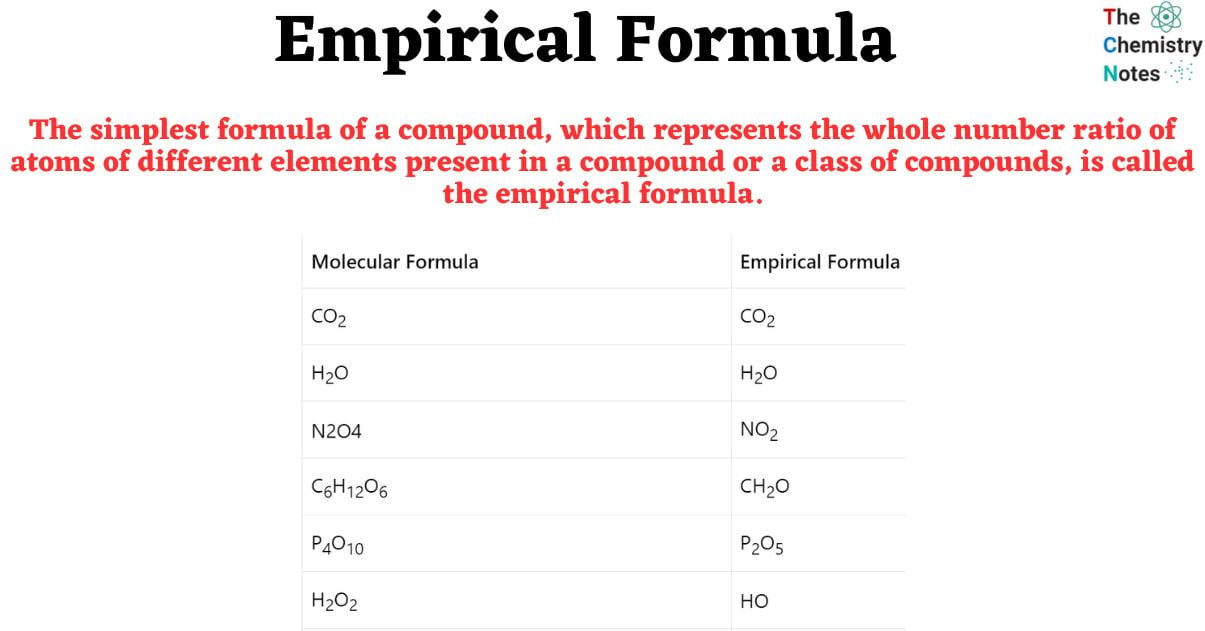
Examples of Molecular and Empirical Formula
| Molecular Formula | Empirical Formula |
| CO2 | CO2 |
| H2O | H2O |
| N2O4 | NO2 |
| C6H12O6 | CH2O |
| P4O10 | P2O5 |
| H2O2 | HO |
| C2H4 | CH2 |
| C6H6 | CH |
| C2H6O2 | CH3O |
Steps to Find Molecular Formula from Empirical Formula
With the use of the empirical formula and molecular weight, you may determine the molecular formula. For example:
Let’s look at hexane as an example. We know that its empirical formula is C3H7 and that it has a molecular weight of 86.2 amu.
Step 1: Determine the molecule’s formula weight first. To achieve this, get the atomic weight of each element in the empirical formula, multiply each one by its subscript, and then sum up all the results to get the formula weight.
You can find the molecular formula from the empirical formula and molecular weight.
Example
For example, let’s find the molecular formula of hexane, knowing its empirical formula is C3H7 and its molecular weight is 86.2 amu.
First calculate the formula weight of the molecule. To do this, look up the atomic weight of each element, multiply each by its subscript in the empirical formula, and then add up all the values to get the formula weight.
- Carbon: 12.01 x 3 = 36.03
- Hydrogen: 1.008 x 7 = 7.056
- Formula weight = 36.03 + 7.056 = 43.09 amu
You now understand that the empirical formula must be multiplied by the molecular formula. By subtracting the empirical weight from the molecular weight, one may get the ratio between the two:
Molecular weight / Empirical weight = 86.2 / 43.09 = 2
Step 2: You’ll frequently receive a decimal value, but it ought to be quite near to an integer. Finally, to obtain the molecular formula, multiply each subscript in the empirical formula by this number:
C3×2 H7×2 = C6H14
References
- https://byjus.com/chemistry/empirical-molecular-formula/
- https://byjus.com/empirical-formula/
- https://www.chem.fsu.edu/chemlab/chm1045/empirical.html
- https://www.thoughtco.com/definition-of-empirical-formula-605084
- Helmenstine, Anne Marie, Ph.D. “Empirical Formula: Definition and Examples.” ThoughtCo, Apr. 5, 2023, thoughtco.com/definition-of-empirical-formula-605084.
- https://www.bbc.co.uk/bitesize/guides/zmgj2nb/revision/1
- https://psiberg.com/empirical-formula/
- https://sciencenotes.org/empirical-vs-molecular-formula/
