The relationship between the reactants and products when a chemical reaction reaches equilibrium is explained by the equilibrium constant of a chemical process, which is typically represented by the letter K. For instance, the ratio of the concentration of the products to the concentration of the reactants, each raised to their respective stoichiometric coefficients, can be used to define the equilibrium constant of concentration (denoted by Kc) of a chemical reaction at equilibrium.
Cato Guldberg (1836–1902) and Peter Waage (1833–1900), two Norwegian chemists, precisely analyzed the components of several reaction systems at equilibrium in 1864.

Interesting Science Videos
What is Equilibrium constant?
The Equilibrium Constant ‘Kc’ is defined as the quantity based on the concentrations of all the distinct reaction species at equilibrium in a reversible reaction.
The equilibrium constant, K, represents the extent of a reaction when it is at equilibrium. For each reactant and product, a ratio is produced using their concentrations and coefficients. We can determine the position of equilibrium by looking at the K value, which also tells us whether a reaction favors reactants or products more.
If K > 1, the equilibrium position is to the right, favoring the reaction’s tendency to create products.
If K < 1, the equilibrium position is to the left, indicating that the production of the reactants is favored.
Similarly, if K = 1, the position of equilibrium is directly in the center, indicating disfavor in increase of neither the products nor the reactants
It is important to keep in mind that temperature affects the value of K and what it says about the reaction. This is due to the fact that a substance’s solubility can change depending on the temperature, which also affects concentrations and, consequently, the equilibrium of a reaction. It is significant to remember that there are several kinds of equilibrium constants that establish relationships between the reactants and products of equilibrium reactions in terms of various units.
Characteristics of the Equilibrium Constant
- It is reaction-specific and fixed at a constant temperature.
- A catalyst affects the rate of both forward and backward reactions in an equal manner, maintaining the equilibrium constant’s value.
- It is possible for changes in concentration, pressure, temperature, and inert gases to have an impact on the equilibrium, favoring either the forward or backward response but not the equilibrium constant.
- Since △G0 = -RT ln K equ, is related to the fundamental free energy.
- Kequ varies depending on the temperature for the same reversible reaction.
- The net equilibrium constant is the product of each stepwise equilibrium constant when numerous stepwise equilibria lead to the end products. Therefore, K = K1 * K2 * K3
- Equilibrium reactions take place simultaneously and have the same result. Reactions’ equilibrium constant does not change. Due to the common product’s larger concentration, product concentrations will decrease.
Calculation of Equilibrium Constant
The equilibrium constant for the equilibrium equation aA + bB ⇌ cC + dD can be determined using the formula;
K = [C]c[D]d / [A]a[B]b, where K is a constant
[C] and [D] are equilibrium product concentrations
[A] and [B] equilibrium reactant concentrations
a, b, c, d are the stoichiometric coefficients from the balanced reaction
The units of concentrations are usually molarity, which has units of molL-1
- All of the product concentrations are in the numerator, and all of the reactant concentrations are in the denominator.
- Each element is then raised to the power of its unique coefficient. The forward reaction rate constant divided by the reverse reaction rate constant gives the equilibrium constant.
- The other names of this relationship is the law of mass action.
- First, according to the law, a chemical reaction’s rate is inversely proportional to the concentrations of the reactants. Additionally, it says that the ratio of reactant to product concentration is constant for a reaction at equilibrium. The equilibrium constant, K, is the name for the constant.
- Additionally, it is important to note that the relationship K = [C]c[D]d / [A]a[B]b is exactly equal to K = kforward / kreverse, where kforward is the rate constant for the forward reaction and kreverse is the rate constant for the reverse reaction:
Rate forward = k forward x [A]a[B]b
Rate reverse = k reverse x [C]c[D]d
Thus, kforward / kreverse = [C]c[D]d / [A]a[B]b = K.
- Additionally, this equation explains why K denotes the degree of reaction. The numerator, which is the concentration of the products, is bigger if K > 1. The denominator, which is the concentration of the reactants, is bigger if K < 1.
Things to Consider during Calculation
- For a certain reaction at a particular temperature, Kc is a constant. Kc fluctuates when the reaction’s temperature is altered.
- The equilibrium statement excludes solvents as well as pure solids and liquids.
- To obtain the right value for Kc, the reaction must be balanced with the coefficients stated as the smallest integer values.
- We can also express the equilibrium constant in terms of the partial pressure of the gases if any of the reactants or products are gases. To distinguish it from the equilibrium constant determined by concentrations in molarity, Kc, we commonly refer equilibrium constant of the partial pressure of the gases as Kp.
Factors Influencing the Equilibrium Constant
- The concentration of excluded reactants or products is released by the reaction in the direction that refills the substance removed.
- Chemical equilibrium modifies the composition of the mixture when the reactant or product concentration varies.
- Pressure changes as a result of changes in volume. If the pressure changes, the total quantity of gaseous reactants and products also changes, changing the gaseous reaction.
- Temperature variations also have an impact on reaction rates. As temperature increases, an exothermic reaction’s equilibrium constant falls.
Types of equilibrium constants
- Using partial pressure (atm) as a measure, gas-phase reactions: Kp
- Water dissociation: The dissociation constant for water; Kw
- Acid dissociation constant; Ka
- Base-water reaction: base hydrolysis constant; Kb
- Precipitation solubility: solubility product; Ksp
- Complex formation: formation constant; Kf
Equilibria in gas reactions: the equilibrium constant, Kp
Partial pressure
It is simpler to quantify pressure than concentration for reactions involving mixtures of gases. A combination of gases is under pressure since each molecule is bombarding the container’s walls. Each gas in the mixture adds to the overall pressure in proportion to the number of moles present when the temperature is constant. Its partial pressure refers to the pressure that any one gas in the mixture is exerting.
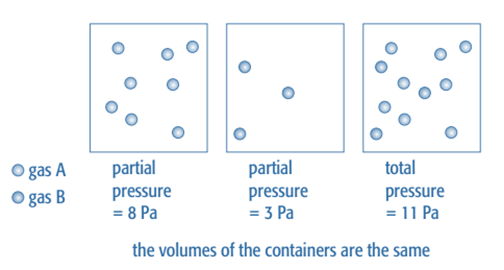
According to the quantity of moles present, each gas in this mixture adds to the pressure.
The sum of the partial pressures of the various gases makes up the overall pressure of a gas.
Ptotal = pA + pB + pC
Where pA, pB, and pC are the partial pressures of the individual gases in the mixture,
Expressions of equilibrium involving partial pressures
We also write equilibrium expressions in terms of partial pressures, similar to how we write equilibrium expressions in terms of concentrations. However, there are a few variations:
- We refer to partial pressure as p.
- Square brackets are not utilized
- we give the equilibrium constant the sign Kp.
- The reactants and products are represented as subscripts after the p
- the number of moles of a given reactant or product is written as power after the p. (the equilibrium constant in terms of partial pressures).
For example, the equilibrium expression for the reaction:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
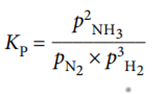
The units of pressure are pascals, Pa. The units of K p depend on the form of the equilibrium expression.
Significance of the equilibrium constant
- There are several applications for the equilibrium constant:
- It can be used to determine the direction a reaction is moving by comparing it to the reaction quotient.
- The reaction’s magnitude can be used to predict how far it will progress until it stops.
- It can be used to determine the relative concentrations of species in an equilibrium system.
References
- P. W. Atkins, Physical Chemistry, 7th Ed.(2002) Oxford University Press, New York.
- https://unacademy.com/content/jee/study-material/chemistry/characteristics-of-equilibriumconstant/#:~:text=The%20equilibrium%20constant%20has%20a,not%20affect %20 the% 20equilibrium%20 constant.
- https://www.studysmarter.us/explanations/chemistry/ physical-chemistry/properties-of- equilibrium- constant/
- https://alevelchemistry.co.uk/definition /equilibrium-constant/
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Text book_Maps/Supplemental_Modules_ (Physical _and _Theoretical_Chemistry)
- https://byjus.com/jee/equilibrium-constant/
