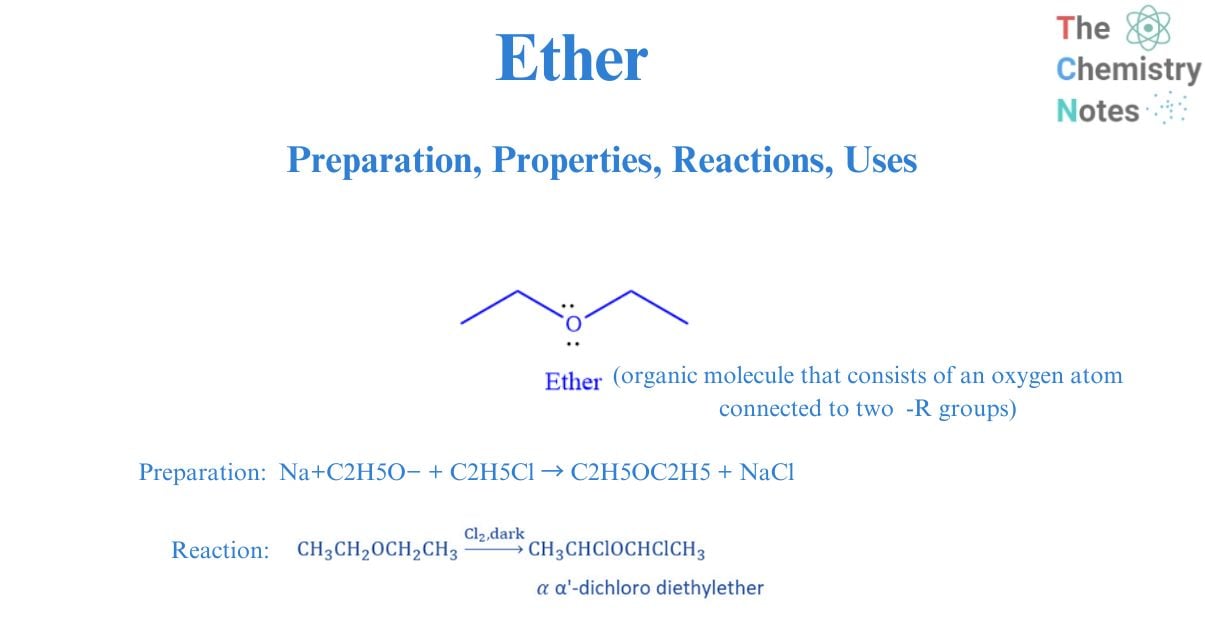
Ethers are a type of organic molecule that consists of an oxygen atom connected to two -R groups. Alkyl, aryl, or vinyl groups can be used as R groups. They are also considered water derivatives in which both hydrogen atoms have been replaced by alkyl or aryl groups. Divalent oxygen in ether is referred to as ethereal oxygen. The term ether is derived from the Latin aether, meaning “to ignite.” Ethers are typically flammable at room temperature and high pressure.
Ethers are less dense than alcohols, less soluble in water, and have lower boiling temperatures. Since they are largely unreactive, they can be used as solvents for fats, oils, waxes, fragrances, resins, dyes, gums, and hydrocarbons. Certain ether vapors are utilized as soil insecticides, miticides, and fumigants.
Interesting Science Videos
Structure of ether
In the C-O-C bond, the oxygen and carbon are sp3 hybridized. The bending shape is caused by the repulsion of two lone pairs (lp) on the oxygen atom. A steric barrier is generated by the existence of bulky groups on both ends of the oxygen atom and the bp-bp repulsion that results in a C-O-C bond angle of approximately 111.7.
Nomenclature of ether
Common name system
In the common name system, Ethers are named by writing the name of two groups connected to the ether oxygen alphabetically, followed by the word ether. For example,
CH3 – O – C2H5 ethyl methyl ether
IUPAC name system
In IUPAC system ethers are named as alkoxyalkanes, alkoxyalkenes, and alkoxyarenes.For example,
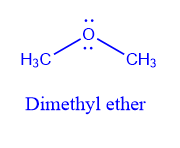
CH3 -O- CH3 Methoxymethane
CH3 -O -C2H5 Methoxyethane
CH3 -O- C6H5 Methoxybenzene
Rules for the IUPAC name system
The longest carbon chain must be chosen.
Change the name of the smaller hydrocarbon group that ends in’yl’ to ‘oxy’.
Before the root or base chain name, put the alkoxy group with a locator number.
Classification of ether
These chemicals are classified into two types or groups. The substituent groups connected to the chemical define this classification. As a result, we can classify them as-
Symmetrical ether: Two identical groups are bonded to one oxygen atom in symmetrical ethers. They are represented as R-O-R or Ar-O-Ar. For example,
Diethyl ether, CH3 – CH2 – O – CH2 – CH3
Asymmetrical ether: In Asymmetrical ether, two distinct groups are linked to a single oxygen atom. They can be represented as R-O-R’ or R-O-Ar. For example,
Ethyl methyl ether, CH3 – O – CH2 – CH3
Preparation of ether
From dehydration of alcohol
Alcohol dehydrates in the presence of protic acids such as phosphoric acids or sulfuric acids. Under various circumstances, it produces ethers and alkenes as reaction products. When ethanol is treated with sulphuric acid at 443K, it dehydrates to ethene. When ethanol is heated with sulphuric acid at 413K, it dehydrates to generate ethoxyethane.
2 CH3CH2−OH+H2SO4 → CH3CH2−O−CH2CH3 + H2O
The dehydration of an alcohol to produce ethers is a nucleophilic substitution process. In this reaction process, one alcohol molecule function as a substrate, while the other acts as a nucleophile. It can use either the SN1 or SN2 mechanism. Secondary and tertiary alcohols, in general, follow the SN1 reaction process. The primary alcohols, on the other hand, use the SN2 reaction mechanism.
From Williamson Ether synthesis
It is a significant approach in laboratories for producing symmetrical and asymmetrical ethers. An alkyl halide is reacted with sodium alkoxide in this technique, resulting in the production of ether. In general, the reaction follows the SN2 pathway for primary alcohol.
Na+C2H5O− + C2H5Cl → C2H5OC2H5 + NaCl
The alkyl halide used in this reaction must be primary. For secondary and tertiary alkyl halides the reaction leads to the formation of alkene.
From silver oxide
The reaction of alkyl halide (R-X) with dry silver oxide (Ag2O), produces ether.
Ag2O + 2 CH3Cl → 2 AgCl + CH3OCH3
Physical properties of ether
- Except for dimethyl ether and ethyl methyl ether, which are gases, all others are colorless, volatile liquids having a pleasant odor at room temperature.
- Lower ethers are extremely volatile and flammable.
- Ethers have polar properties. Ether’s dipole moment is the vector sum of two polar C-O bonds, with a large contribution from two lone electron pairs. Diethyl ether, for example, has a dipole moment of 1.18D.
- As ether’s oxygen can form hydrogen bonds with water, they are miscible with water. Ethers dissolve both polar and non-polar compounds.
- Ethers have significantly lower boiling points than alcohols. This is because alcohols can form intramolecular hydrogen bonding, While in ethers, the hydrogen atom is not directly bonded to the oxygen atom. As a result, there are no hydrogen bonds between molecules in ethers. The boiling points of ethers are slightly higher than those of alkanes.
- Ethers containing up to three carbon atoms are miscible in water. This is because smaller ethers establish hydrogen bonds with water molecules more easily. As ethers consist of nonpolar hydrophobic hydrocarbon chains and polar hydrophilic oxygen, the solubility of ether in water decreases with an increase in the size of the alkyl group.
- The oxygen bonds in water, ethers, and alcohol are all similar. As oxygen is more electronegative than carbon, the hydrogen atoms in simple hydrocarbons alpha to the ethereal oxygen, are more acidic.
Reactions of ether
Ethers are the least reactive functional group, so ether bonds are highly resistant to bases, oxidizing agents, and reducing agents. Some important reactions of ethers are:
Reaction involving C -O bond
Cleavage of C – O – C bond
Under suitable conditions, cleavage of C-O bonds in ether occurs in excess hydrogen halide.
The reactivity of hydrogen halides is in the order of:
HI > HBr > HCl
The produced alkyl halide is invariably of the lower alkyl group. if ethers contain a tertiary alkyl group, the cleavage always produces tertiary alkyl halide. In the case of phenolic ethers, cleavage results in the production of phenol and alkyl halide.
R -O- R’ + HX → R – X + R’OH
Combustion reaction
Because of their high combustibility, ethers produce water and carbon dioxide when they come into contact with air.
C2H5OC2H5 + 6O 2 → 4 CO2 + 5 H2O
Reaction due to alkyl group
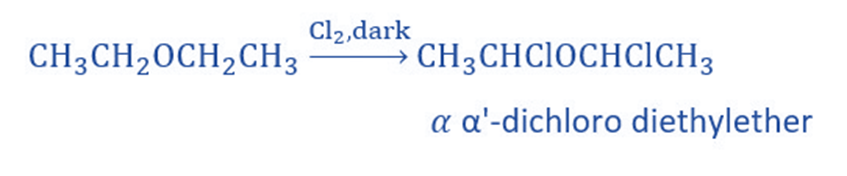
Halogenation
In the absence of sunlight, the alkyl group of aliphatic ether undergoes substitution with chlorine or bromine, resulting in alpha-halogenated ethers.
Electrophilic reaction
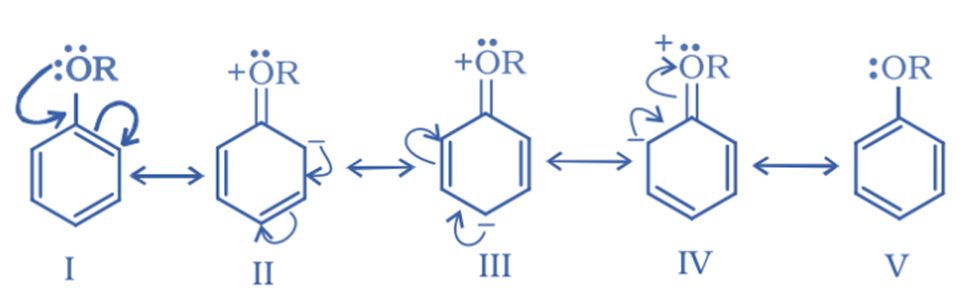
Due to the presence of alkoxy groups (-OR), aromatic ethers activate their aromatic ring towards an electrophilic substitution process. This alkoxy group has ortho and para-directing properties. The lone pair of oxygen in aryl ethers is in resonance with the benzene ring, increasing electron density in the ring at ortho and para locations. This, in turn, enhances electrophile attack at ortho and para positions.
Halogenation
Phenylalkyl ethers can undergo typical benzene ring halogenation; for example, even in the absence of a catalyst, anisole undergoes bromination with bromine in ethanoic acid. It is caused by the methoxy group activating the benzene ring.
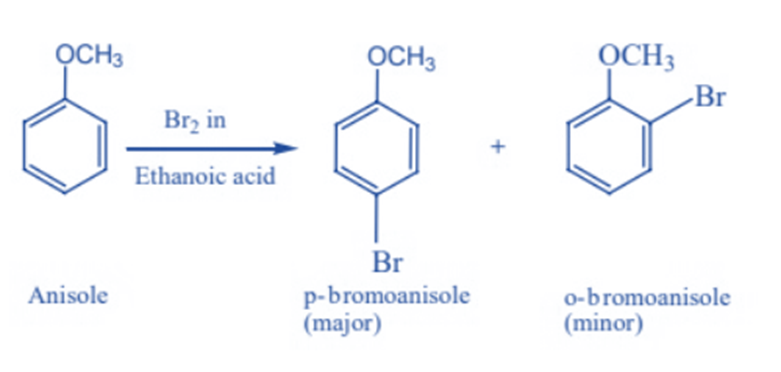
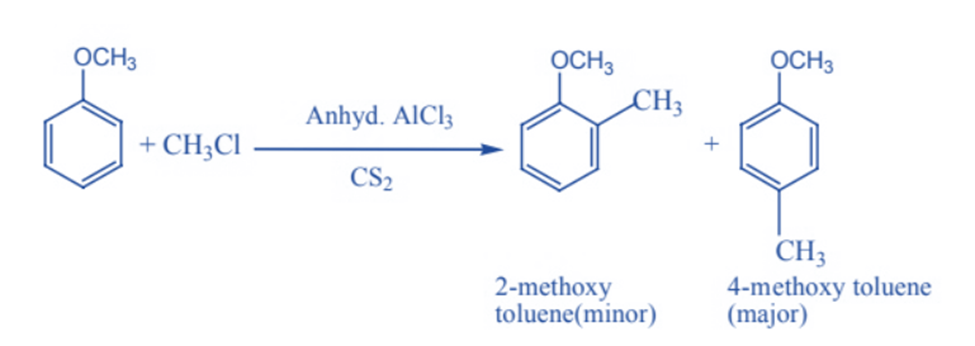
Friedel craft alkylation
In the presence of anhydrous aluminum chloride (a Lewis acid) as a catalyst, anisole interacts with an alkyl halide to produce 2-methoxy toluene and 4-methoxy toluene. In this reaction, alkyl are introduced at ortho and para positions.
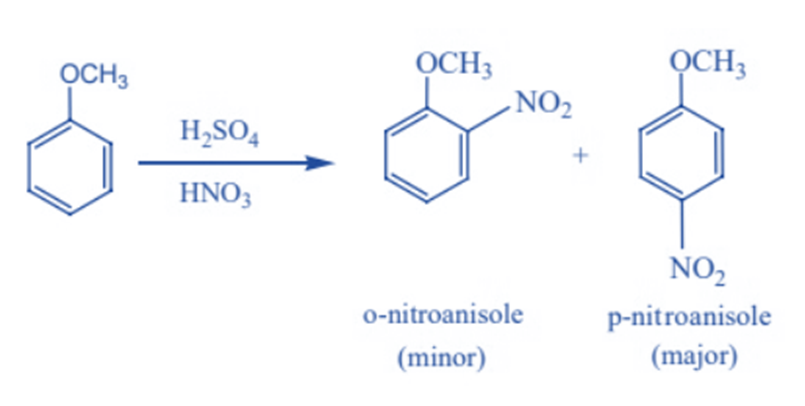
Nitration
Nitration of anisole with a mixture of strong nitric (HNO3) and sulphuric (H2SO4) acids produces a mixture of ortho- and para-Nitroanisole (major).
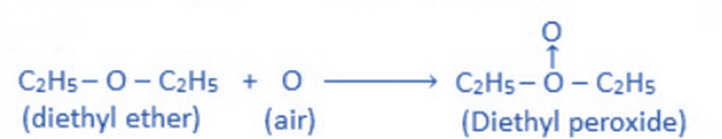
Reaction due to ethereal oxygen
Peroxide formation
In the presence of sunlight, ether reacts with atmospheric oxygen to produce ether peroxide due to the presence of a lone pair of electrons on the ethereal oxygen.
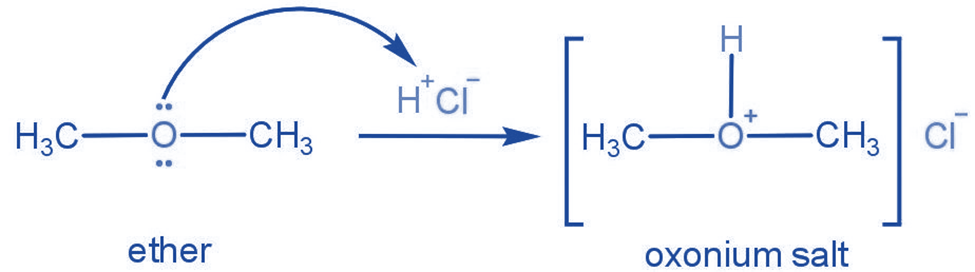
Formation of Oxonium salt
Ethers can act as weak Lewis bases. They form oxonium salts when they are dissolved in cold, strong mineral acids like hydrochloric or sulfuric acid.
Uses of ether
- They are used as the starting material for various organic synthesis reactions.
- They are used as organic solvents and are also used to dissolve oil, resin, gasoline, gum, and other things.
- Ethers are also useful in medicine and pharmacology, particularly as anesthetics. Ethyl ether (CH3CH2OCH2CH3), sometimes known simply as ether, was initially used as a surgical anesthetic in 1842.
- At low temperatures, dimethyl ether is employed as a refrigerant and a solvent.
- Since phenyl ether has a high boiling point, it can be utilized as a heat transfer fluid.
- They are also utilized as a reaction medium for numerous operations, such as the production of the Grignard’s reagent, the Wurtz reaction, and so on, due to their nearly inert nature and excellent dissolving power.
- The methyl ether of morphine, i.e., codeine, is a powerful pain reliever.
- Certain ether vapors are utilized as insecticides, fumigants, and miticides.
- Higher molecular weight ethers can be employed as solvents for varnishes and lacquers.
- Dimethyl ether acts as a propellant and is used in aerosol sprays.
- Ethers provide wonderful muscular relaxation, pulse rate, and respiratory rate.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Arun Bahl, and B.S. Bahl (2006). A textbook of organic chemistry Chemistry. New delhi: S. CHAND.
- https://byjus.com/jee/ethers/#:~:text=Ethers%20are%20a%20class%20of,pressure%2C%20ethers%20are%20usually%20flammable.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/18%3A_Ethers_and_Epoxides_Thiols_and_Sulfides/18.01%3A_Names_and_Properties_of_Ethers.
- https://faculty.ksu.edu.sa/sites/default/files/8-_145_chem-ethers.pdf.
- https://www.vedantu.com/iit-jee/ethers.
