The Faraday’s constant, F, is the physical constant equal to the total electric charge carried by one mole of electrons. The constant is named after English physicist Michael Faraday. The Faraday constant is commonly utilized in electrolysis calculations. We use Faraday’s constant to compute the amount of electric charge that travels across a circuit per unit of time. The Faraday constant is equal to the electric charge per mole of elementary charges (such as electrons). One mole is defined as the number of atoms or molecules in exactly 12 grams of pure carbon-12. Avogadro’s number (NA) is roughly 6.022 x 1023 atoms or molecules.
Interesting Science Videos
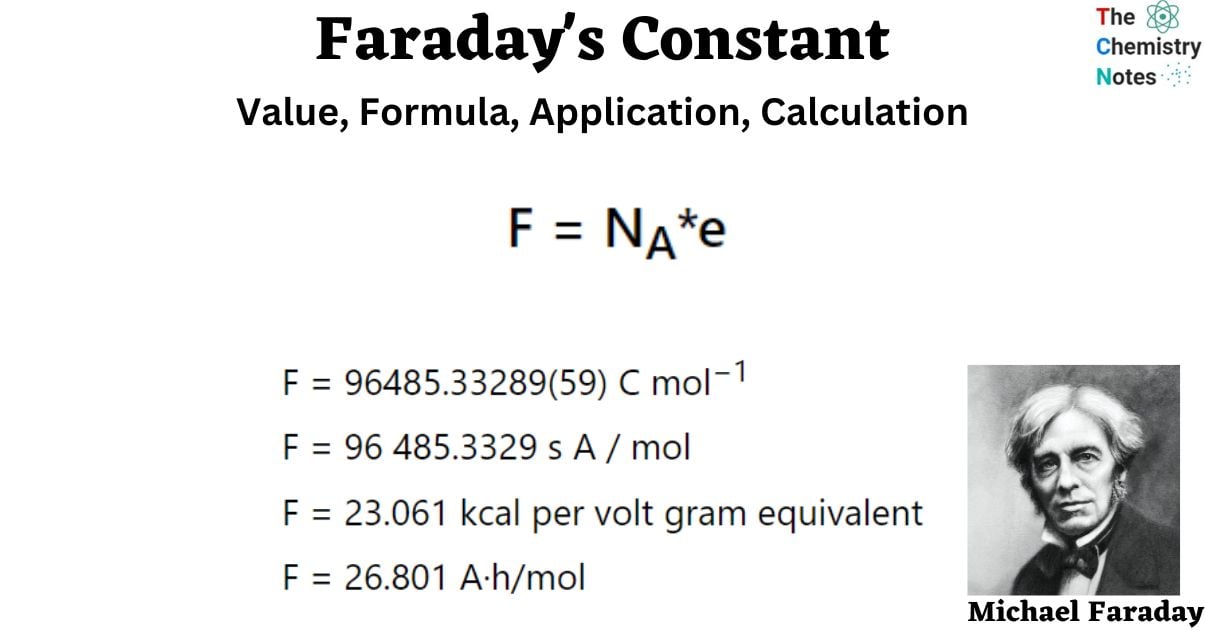
Faraday Constant Formula
F = NA*e
Where:
- F = Faraday’s constant
- NA = Avogadro’s constant (approximately 6.022 x 1023 electrons per mole)
- e = the charge of a single electron (approximately 1.602 x 10-19 Coulombs)
As a result, the Faraday constant is defined as the charge of one mole of electrons multiplied by Avogadro’s number (NA) of electrons.
Faraday’s Constant Value
The values for faraday’s constant are:
- F = 96485.33289(59) C mol−1
- F = 96 485.3329 s A / mol
- F = 23.061 kcal per volt gram equivalent
- F = 26.801 A·h/mol
Calculation of Faraday’s Constant
Given that 1 mole electron equals 6.022*1023 electrons (Avogadro’s number) and 1 coulomb equals the (negative) charge of 6.241*1018 electrons, the Faraday constant is:
F = 96485.33289(59) C mol−1
F was initially estimated by measuring the quantity of silver deposited in an electrochemical process that passed a measured current for a defined duration and then applying Faraday’s equation of electrolysis to the resultant weight.
The Faraday constant has been exactly specified as a result of the 2019 redefinition of the International System of Units (SI), which included precisely defined values for the elementary charge and the mole.
Charge of 1 mole in coulomb
=1.602176634×10−19C × 6.02214076×1023
=96485.33212331…C
=96485.3321C
=96500C
Application of Faraday’s Constant
The Faraday constant is employed in many fields, including electrochemistry, thermodynamics, battery technology, analytical chemistry, and so on.
- Faraday Constant is commonly used in physical chemistry. The charge carried by one mole of electrons is represented by the Faraday constant, denoted by the symbol F. Consider it the coulomb’s and mole’s conservation factor. Because of this, it is used in electrochemistry.
- Faraday’s constant is used in electrochemistry to calculate the amount of electric charge involved in a chemical reaction, as well as the electrochemical equivalent of a substance. The relationship between the amount of electric charge going through an electrolytic cell and the amount of material electrodeposited or dissolved at the electrodes is calculated using this constant.
- Faraday’s constant is used in the theoretical study of electrochemical cells to compute the potential difference between the electrodes and the amount of material involved in the redox reactions.
- Faraday’s constant is crucial in determining battery capacity. The constant is used to compute the amount of charge required to produce one mole of a chemical, which is useful in calculating the amount of energy stored in a battery.
Who was Michael Faraday?
Michael Faraday (22 September 1791 – 25 August 1867) was an English scientist who made substantial contributions to the disciplines of electrochemistry and electromagnetics. He was born and died in London. Among his most noteworthy contributions are the ideas that underpin electromagnetic induction, diamagnetism, and electrolysis.
Frequently Asked Questions (FAQ)
What is the Faraday Constant’s value?
It is now the most widely recognized value.
Who discovered the Faraday Constant?
Michael Faraday discovered the Faraday Constant.
What is the formula of the Faraday Constant?
This constant may be written as F = e NA in terms of two additional physical constants.
Where e is the electron’s charge in coulombs, e = 1.60217662*10-19 C
The Avogadro constant is denoted by NA. NA = 6.022141×1023 mol-1.
What is the use of Faraday’s constant?
One of the most common applications of the Faraday constant is electrolysis. Divide the quantity of charge in coulombs by the Faraday constant to get the number of oxidized elements.
Video on Faraday Constant
References
- https://testbook.com/physics/faraday-constant
- Helmenstine, Anne Marie, Ph.D. “Faraday Constant Definition.” ThoughtCo, Aug. 27, 2020, thoughtco.com/definition-of-faraday-constant-605120.
- https://www.collegesearch.in/articles/faraday-constant
- https://byjus.com/physics/faraday-constant/
- https://collegedunia.com/exams/faraday-constant-value-definition-equation-examples-physics-articleid-899#c1
- https://unacademy.com/content/jee/study-material/physics/faraday-constant/
- https://builtin.com/hardware/faraday-constant

