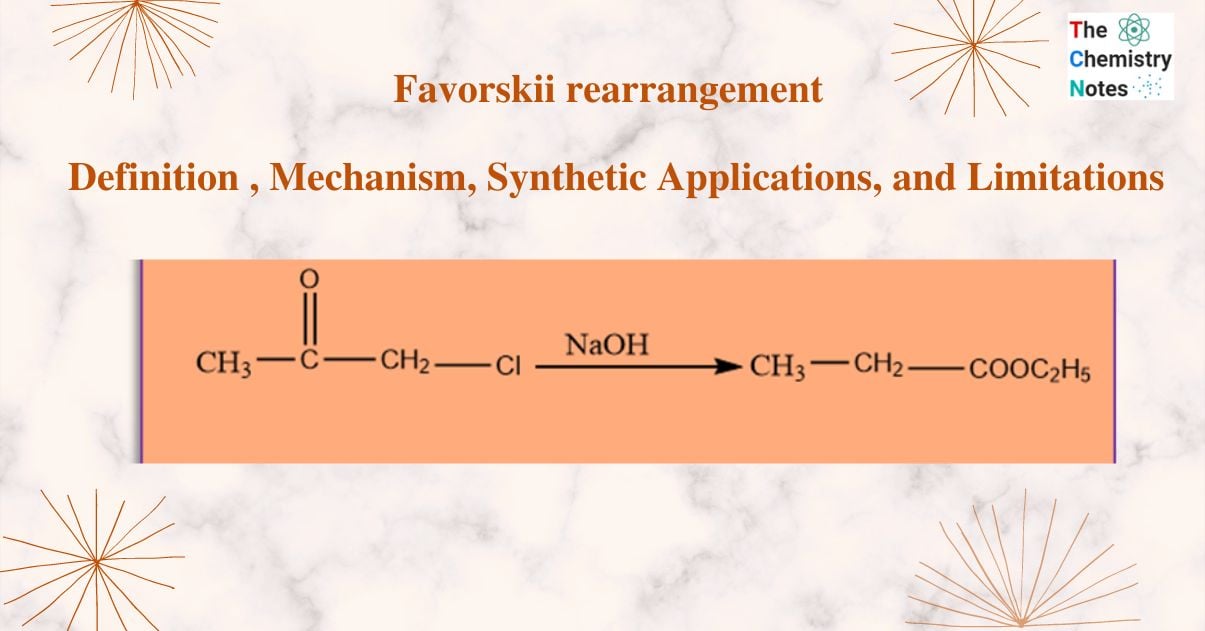
Favorskii rearrangement involves the conversion of cyclopropanones or α -halo ketones to carboxylic acids or their derivatives in presence of the base. Acidic “‘ α -hydrogens” must be present in the halo ketones.
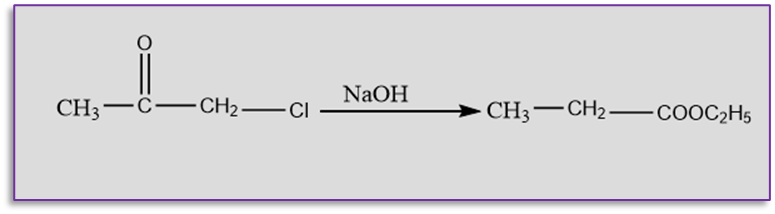
This reaction is named after the Russian scientist Alexei Yevgrafovich Favorskii,. The reaction primarily involves the rearrangement of α -halo ketones to produce carboxylic acid derivatives. Ring contraction arises from the Favorskii rearrangement of α -halo cyclic ketones. In this reaction, if alkoxides are utilized as bases esters are produced. However, when amines are utilized as bases, It gives amide as the final product. The reaction in acyclic systems functions best when the carbon atom carrying the halide is substituted. Depending on the structure of the -halo ketone, the requirements for the rearrangement may change.
Interesting Science Videos
Reaction mechanism of Favorskii rearrangement
The production of an enolate on the side of the ketone opposite the chlorine atom is the first step in the reaction process. This enolate undergoes cyclization to give a cyclopropanone intermediate. The result is the rearranged ester after the cyclopropane intermediate has undergone subsequent OH- addition, ring opening, and proton exchange to produce the most stable carbanions.
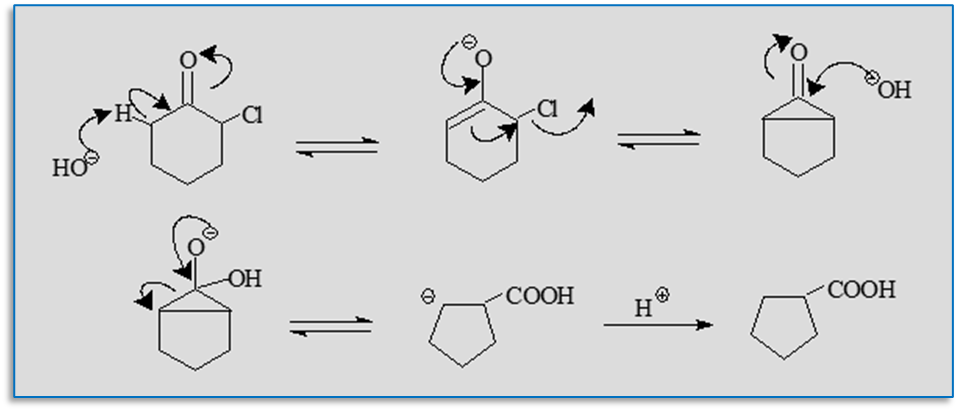
When the production of enolates is difficult, the Favorskii rearrangement occurs via a different mechanism. In this reaction process, When ketone is combined with hydroxide, a coordinated collapse of the tetrahedral intermediate and the migration of the adjacent carbon with the displacement of the halide occur. Although before labeling studies, it was believed that all Favorskii rearrangements happened via this mechanism. So, this process is also referred to as the pseudo-Favorskii rearrangement.
Synthetic applications
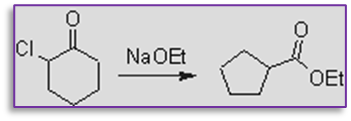
1. This provides effective method for the conversion of alpha halo ketone into carboxylic acid. for example, by treating with sodium ethoxide, 2-chlorocyclohexanone can be changed into ethyl cyclopentane carboxylate. in this reaction, a six-member ring is contracted to form a five-member ring.
2. Bicyclic esters can be synthesized via this process.
3. The favoroskii rearrangement step is also involved in the synthesis of cubane, as seen below.
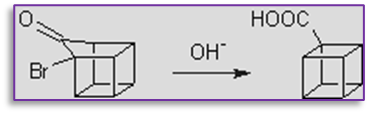
4. This reaction can be used to prepare both steroids and nonsteroids.
5. It is especially beneficial in the synthesis of branched-chain aliphatic carboxylic acids and their derivatives, as well as in the ring contraction of cyclic ketones.
Limitations
Due to their sensitivity, -halo ketones can produce a variety of undesirable side products. These may include α-alkoxy ketones, unsaturated ketones, and epoxyethers (primarily from aryl substituted alpha halo ketone), as well as products obtained from them.
References
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/favorskii-rearrangement
